
Providing courses, products & services so Clinical Research Professionals can thrive & achieve fulfilling careers.

What is the Difference Between a CRC and a CRA?
by ClinEssentials Team | Oct 4, 2023 | Clinical Research Careers , Clinical Research Associates , Clinical Research Coordinators , New to Clinical Research , Tips for Research Professionals | 3 comments

When you start a new job, decide on a career path, or want to learn more about an industry, the job titles, acronyms, and industry-specific language can be confusing!
This certainly holds true in Clinical Research. For job titles alone, we often hear CRA, CRC, CTM, CTA, PI, Sub-I, DM, and many other acronyms, which can overwhelm a new research professional.
Beginning with the acronyms, CRC stands for Clinical Research Coordinator, and CRA stands for Clinical Research Associate. The titles sound as though they could be interchangeable, but knowing the job duties and qualifications will make it easy to differentiate between the roles.
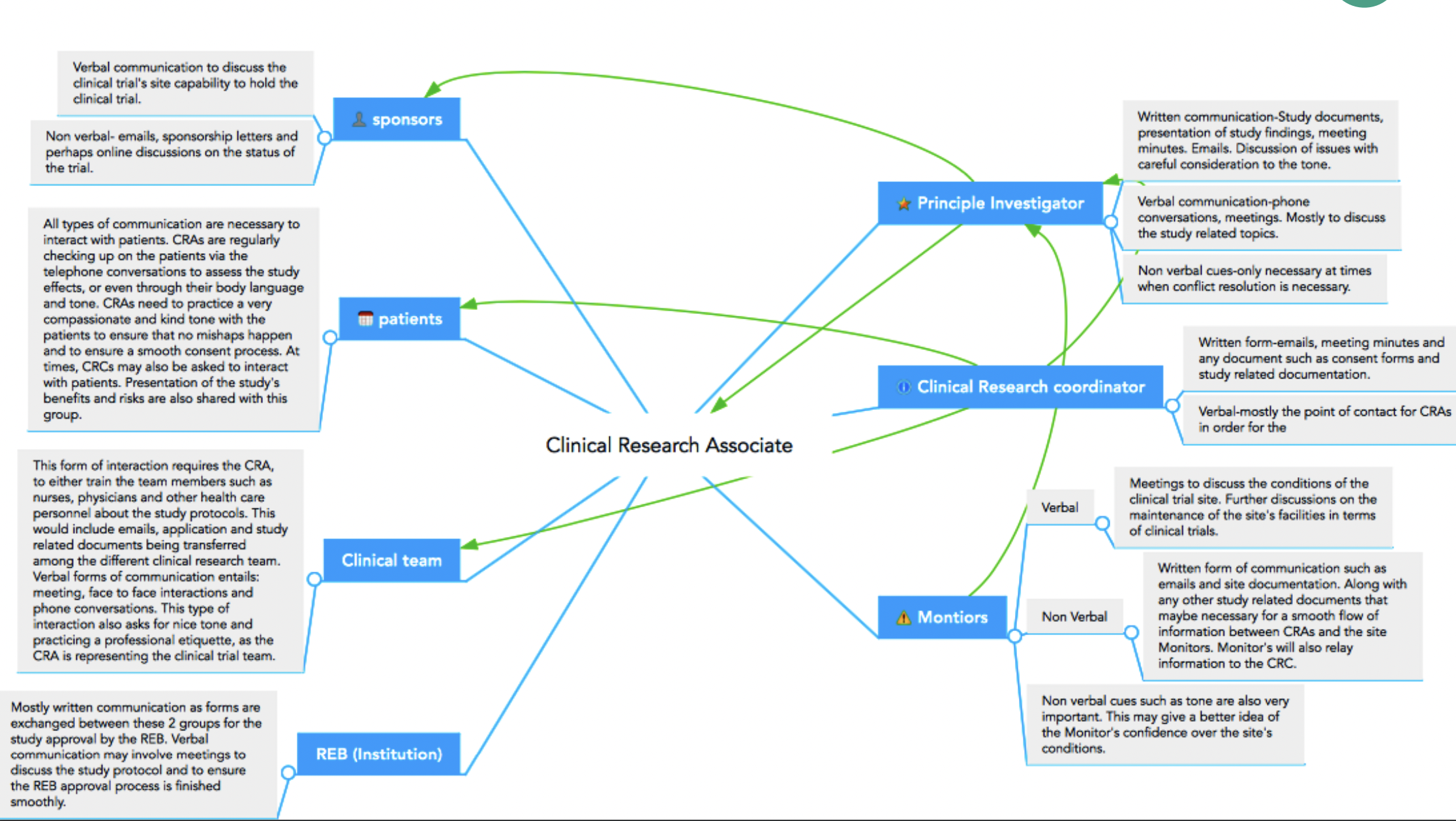
CRCs and CRAs work together on a research team, and although their roles sound similar, there are profound differences, including educational requirements, patient involvement, and salary.
Since CRC and CRA are two job titles in Clinical Research that you hear about most and need clarification on, this article will discuss each role and what makes them different.
What does a CRC do?
A Clinical Research Coordinator, also called a Clinical Trial Coordinator or Study Coordinator, works at a research site under the Principal Investigator’s (PI) supervision. CRCs engage and work with patients and directly support, facilitate, and organize clinical trial activities across one or more trials at their site.
Characteristics of successful CRCs include exceptional communication and interpersonal skills, the ability to multi-task and stay organized, and being naturally analytical, willing to learn, curious, and empathetic.
Those who exhibit all or most of these personality traits may be better equipped to take on the key responsibilities that come with the CRC role, which typically include the following:
- Planning and managing the study by creating source documents, maintaining organization, filing, etc.
- Enrolling patients in the study by finding qualified individuals, sharing study information, providing answers to questions, and performing study visits according to the protocol, including laboratory sample collection and processing
- Maintaining patient enrollment by building relationships, communicating, following up, answering questions, and being the patient’s primary contact
- Training staff or patients on study-specific parameters and other important study-related procedures
- Maintaining accurate records of study drugs and study supplies
- Performing data entry into study-specific systems
- Staying in compliance with the study protocol as well as federal, state, and institutional regulations
- Working closely with cross-functional teams involved with the study and the CRA, who in turn works with the Sponsor or Clinical Research Organization (CRO)
The Association of Clinical Research Professionals (ACRP) created a list of Core Competency Guidelines for Clinical Research Coordinators to use for self-assessment, analyzing gaps in competence, and creating professional development plans.
Clinical Research Coordinators generally do not travel. Most CRC roles are in-person at a research site. There are various levels of the CRC role that are typically based on years of experience. Each company is different, but titles may include Assistant CRC or Research Assistant, CRC 1, CRC 2, and Lead CRC. A natural career progression for a CRC is to the CRA role.
PRO TIP: If you are interested in learning more about the Clinical Research Coordinator job, a straightforward way to find information is by reviewing job descriptions on a job search website like Indeed .
What does a CRA do?
Clinical Research Associates (CRAs) are the liaison between a Sponsor or CRO and the research site(s) for the clinical trial.
CRAs play a crucial role in clinical trial monitoring, which is when a CRA visits a site to interact with the PI and Clinical Research Coordinator and confirm the study protocol and other regulations are being followed. A monitoring visit is jam-packed as the CRA is responsible for reviewing patient data and site study files, confirming patients meet eligibility requirements, inventorying study supplies, including study drugs, answering questions from the site, implementing action plans and training (if needed), and much more.
CRAs do not interact with trial patients.
Along with monitoring, other critical parts of the CRA’s role are:
- Documentation
- Organization
- Effective communication
- Ability to problem-solve
Most Clinical Research Associates are involved with multiple clinical trials at a time and can manage 10 – 15 sites on average per study. A CRA’s typical daily responsibilities differ depending on the clinical trial stage. In this article , you can read more about what a CRA does during each phase.
The best CRAs are excellent communicators with top-notch interpersonal skills, excel at relationship building, are highly motivated and good at motivating others, and are detail-oriented, organized multi-taskers.
The ACRP created this Core Competency Framework for Clinical Study Monitoring that CRAs can use as a reference for their job expectations and performance.
CRAs generally travel to assigned sites for monitoring approximately eight to ten days per month. Most CRAs work out of a home office when they are not traveling.
There are several levels of the CRA role, and they vary by company. Common titles include CRA 1, CRA 2, Senior CRA (Sr. CRA or SCRA) 1, Sr. CRA 2, Principal CRA, Lead CRA (LCRA), and In-House CRA (IHCRA).
- A Principal CRA typically oversees the CRAs assigned to a project while maintaining all their CRA responsibilities. A person may take on this role if they are not interested in becoming a Clinical Trial Manager (CTM).
- A Lead CRA often takes on some CTM duties but also retains some of their CRA responsibilities. LCRA is a hybrid role between the CRA and CTM, but an LCRA is interested in becoming a CTM in the future, so this role serves as a bridge. A person in the LCRA role may be a trip report reviewer, the lead CRA trainer, a point of escalation for the CRAs when traveling, a reviewer of study metrics, and a chair of CRA team meetings.
- An In-House CRA does not travel and usually works for a Sponsor or CRO in a more administrative role. A person in this role typically supports traveling CRAs by assisting with action item closure and site follow-up and may be gaining experience to become a CRA or CTM.
A natural career progression for a CRA is to the Clinical Trial Manager role.
What are the differences between the CRC and CRA roles?

The Clinical Research Coordinator and Clinical Research Associate roles are different. However, because there can be some overlap, here are four key differences between a CRC and a CRA.
- CRCs and CRAs have different educational requirements.* A Clinical Research Coordinator can be an entry-level position. A high school diploma or GED is required for the CRC role. Zero to two years of Clinical Research experience is ideal but not always mandatory at some research sites. A college degree is not a requirement; however, a four-year bachelor’s degree in a health-related field may be helpful for CRCs who want to continue to level up in the industry. Knowledge of medical terminology, Good Clinical Practice, ALCOA-CCEA, and ICH Guidelines are essential for a CRC. A Clinical Research Associate is not an entry-level position within the Clinical Research industry. A Bachelor’s degree in a health-related field, including clinical research, health science, or biological science, is typically preferred. In addition, a minimum of 1-2 years of field-based monitoring or other relevant experience is a requirement of CRAs by most employers. A natural career progression for a Clinical Research Coordinator is to the Clinical Research Associate role. Experienced CRAs often transition to the Clinical Trial Manager role. *The information in this section is typical for CRCs and CRAs, but requirements may vary between companies.
- Employment specifications for CRCs and CRAs are not the same. CRCs work for a research clinic at the site level and typically report directly to the Principal Investigator (PI). CRCs work in person at the research clinic or trial site. CRAs are often employed by a Pharmaceutical Company or through a Clinical Research Organization (CRO). In addition, some CRAs work as independent contractors. CRAs commonly work from a home office and schedule monitoring visits with their assigned sites. Another difference is the working relationship with the PI. For example, the PI supervises the CRC, whereas the CRA’s job is to ensure the PI complies with the research protocols.
- CRCs and CRAs have different roles with trial participants and data collection. Clinical Research Coordinators interact and work with patients directly. CRCs are involved with recruitment, scheduling patient visits, and collecting patient data. CRAs do not interact with trial participants but ensure that enrollment standards are met. Rather than collecting data, CRAs monitor the data collected with a focus on data quality and patient safety.
- The salary for CRCs and CRAs can be a differentiating factor. In general, a CRA typically receives a higher salary than a CRC. Of course, there are exceptions, but due to a Clinical Research Associate’s educational and travel requirements, their income is generally higher than that of a Clinical Research Coordinator. Many individuals join the Clinical Research industry as CRC, and the increased salary is a motivating factor when trying to move to the CRA role.
What are the similarities between the CRC and CRA roles?
The most important similarity between a Clinical Research Coordinator and a Clinical Research Associate is that both roles are essential to a research study. Their involvement is integral to the success of a clinical trial.
CRCs and CRAs must be organized, effective communicators who are good at multitasking and passionate about Clinical Research.
CRCs and CRAs are often called the “backbone of a clinical study.” While both are valued roles that carry intense responsibility, the focus is different: CRCs focus on patient-related activities, and CRAs focus on study monitoring.
As Clinical Research team members, CRCs, and CRAs can help increase their teams’ efficiency and lead them to more studies that conclude on time, within budget, and produce quality results.
Many CRCs and CRAs love using the time-saving tools and resources from ClinEssentials to help them do their job well in less time – take a look at the Shop !
Next Steps for Aspiring and Current CRCs and CRAs
If you want to enter the Clinical Research industry with an entry-level role, the Clinical Research Coordinator role may be something to read more about. If you have some experience, the Clinical Research Associate role may be a good fit. If you want to become a Clinical Trial Manager, download this FREE guide about transitioning to the CTM role.
While you can find plenty of information about CRCs and CRAs online, talking to someone with industry experience is sometimes more helpful. ClinEssentials has a Career Services program designed to help with career advice, professional development, resume reviews, and more! Click here to schedule a consultation .
What other questions do you have about CRCs versus CRAs? What other Clinical Research roles would you like to learn more about? Please comment below!
Tiffany Ashton, MAS, CCRA, has over twenty years of experience as a Clinical Research Professional. Tiffany is the Director of Operations for ClinEssentials, a consultant in the Clinical Trial Manager role, and the expert instructor for the CTM Training Course.
Thank you for the information, I highly interested in becoming a CRA however it’s difficult if you don’t have experience. Do you conduct CRA training in your company?
We do not offer CRA training at this time, however, we highly suggest the following guidebook to help you navigate your Clinical Research career. It has links to trusted CRA training programs amongst other important facts to know about the industry: https://clinessentials.com/intro-clinical-research-guidebook/
“Thanks for the dose of positivity!”
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Submit Comment
More Resources

5 Tips for Clinical Trial Managers (CTMs) When Planning for First Site Activated
by ClinEssentials Team | Sep 18, 2024 | Clinical Trial Managers

The Importance of the Trial Master File for Each Clinical Trial – and Why Teamwork is Vital
by ClinEssentials Team | Jul 10, 2024 | Tips for Research Professionals

How to Get into Clinical Research
by ClinEssentials Team | Feb 7, 2024 | Clinical Research Careers , Clinical Research Coordinators , Clinical Trial Assistants , Tips for Research Professionals

How Clinical Trial Managers Are Involved with Study Recruitment
by ClinEssentials Team | Jan 10, 2024 | Clinical Research Careers , Clinical Trial Managers , Tips for Research Professionals

Clinical Research Associate vs Coordinator (CRA vs CRC)
What is a cra/crc.
Use this guide to get a detailed side-by-side comparison of two similar acronyms with 2 very different roles.
The clinical research coordinator or CRC helps conduct the trial as one specific site and will archive all the documents at the site when the verification by CRA is complete.
The clinical research associate or CRA will review and verify documents from multiple sites conducting the same trial and do multiple visits to ensure quality and ethical conduct of the clinical trial .
CRA vs CRC (Clinical Research Coordinator vs Associate)
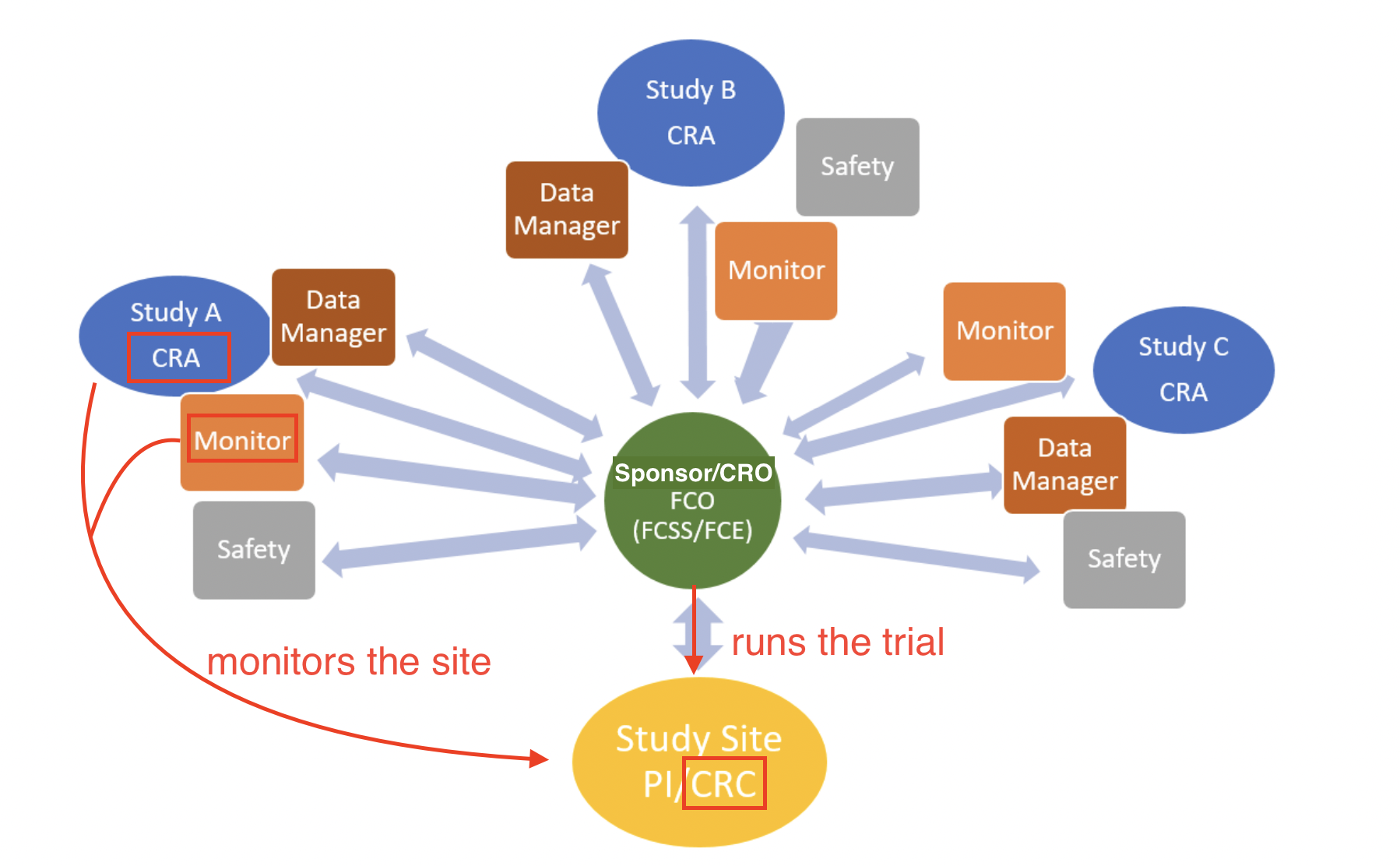
Clinical Research Associate vs Coordinator
Difference between clinical research associate and coordinator:
A clinical research associate ( CRA ), also called a clinical monitor or trial monitor , is a research professional with a minimum of a bachelors degree (usually nurses!) who works under contracts or hired by sponsors, CROs, or freelancing (by biopharma and research institutes) to perform roles listed in ICH GCP guidelines for monitoring clinical trials.
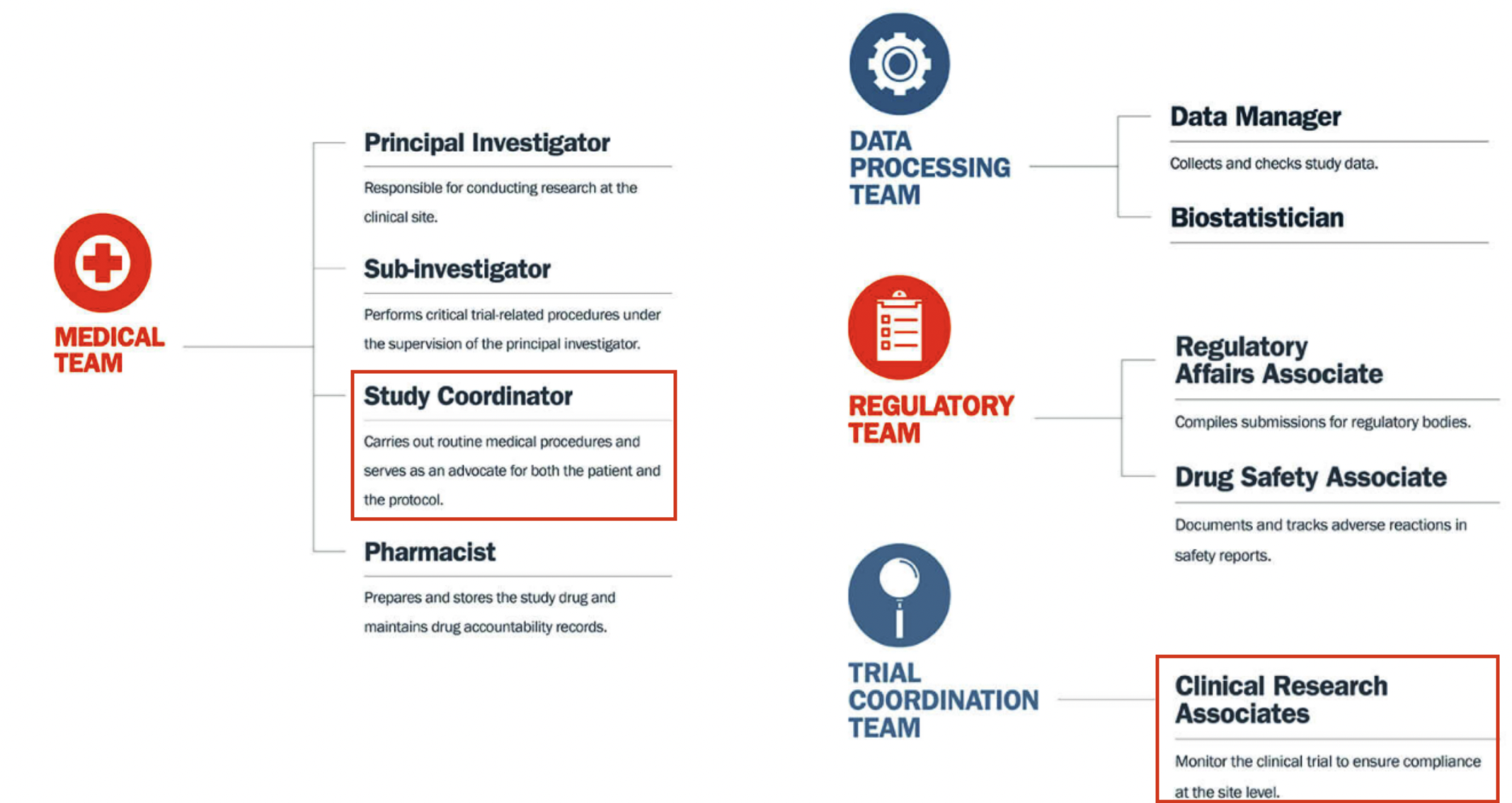
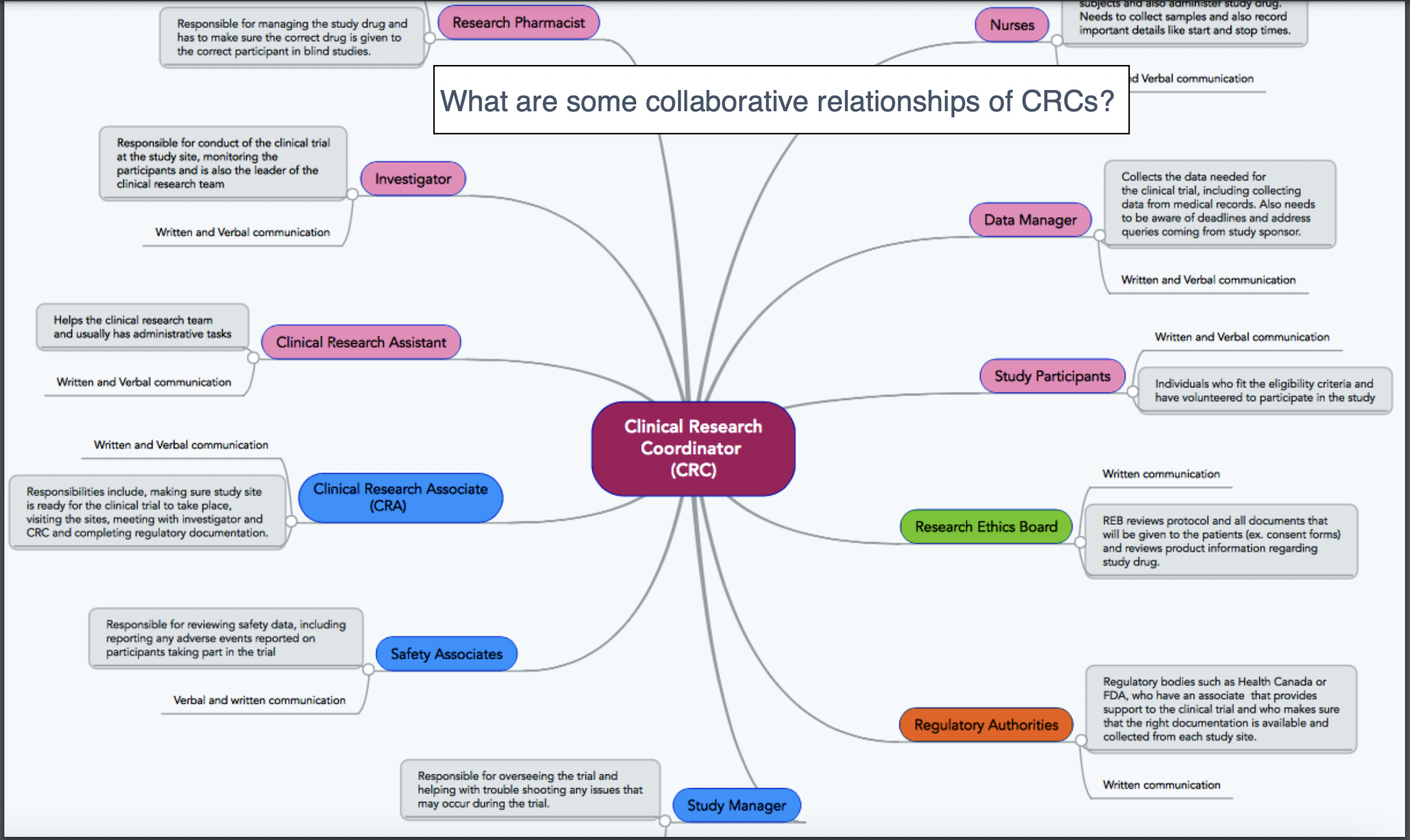
Who makes up the research team?
A CRA ensures compliance with ICH GCP and the clinical trial protocol by checking clinical site activities, making on-site visits (selection, initiation, routine, close-out), verifying “trial” case report forms (CRFs) are accurate by comparing to medical records, and speaking with the site’s CRC.
CRAs protect the ethical safety of human subjects and ensure the scientific integrity of the data collected through these processes.
Difference between clinical research coordinator and clinical research associate:
When a PI (principal investigator, i.e. often a busy, working physician running a trial on the side) is chosen to conduct a trial at their site, clinical research coordinators often take over part of the essential responsibilities of PI. This includes making sure the trial is 1) conducted and 2) in compliance with the protocol and federal or international regulations.
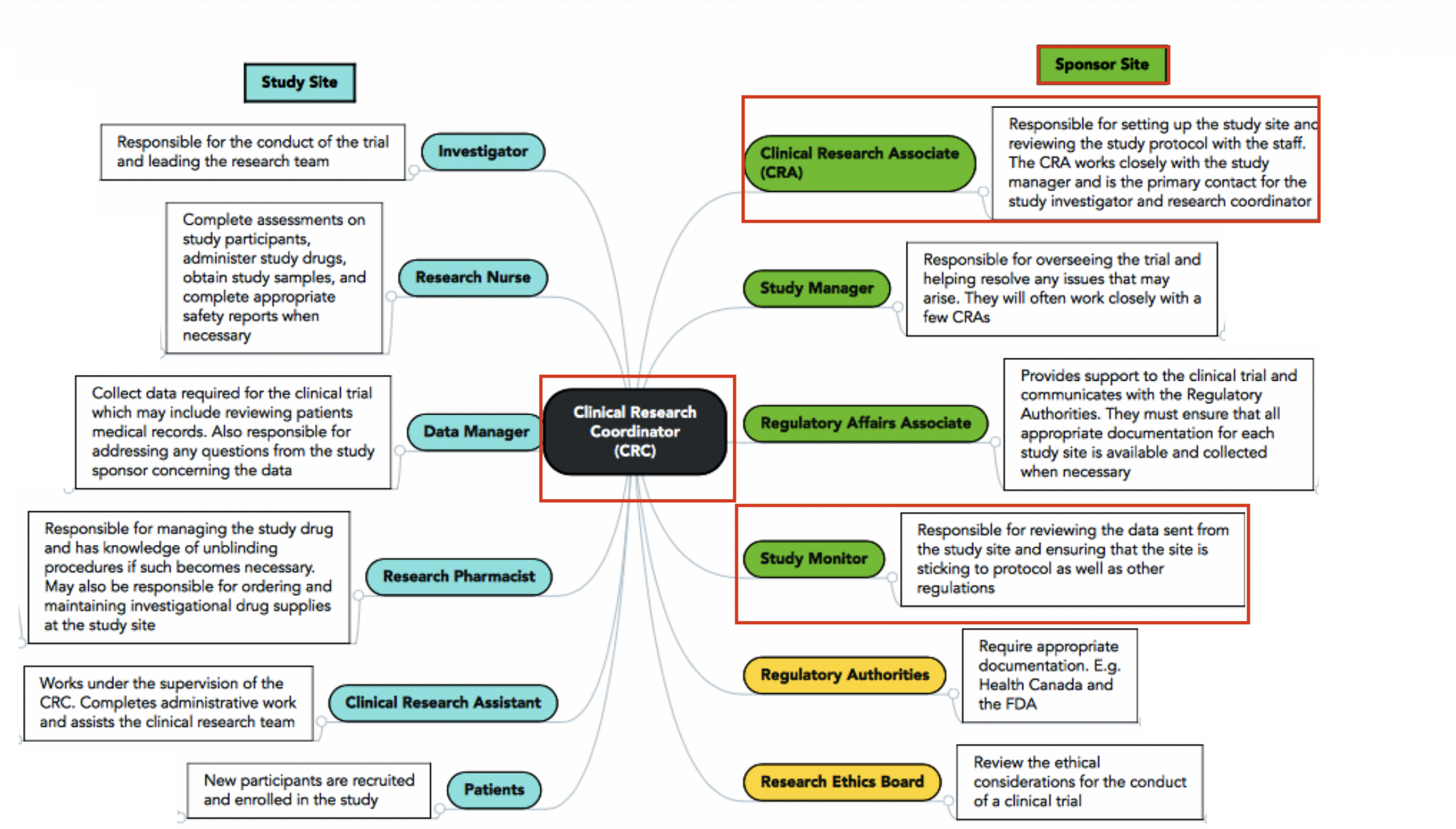
Site (CRC) vs. Sponsor (CRA)
CRC vs CRA: CRC responsibilities include writing the IRB/Ethics Committee application (specific to each site unless trial is under a single IRB/sIRB), making/performing informed consent (IC) process, developing a budget for the site, subject recruitment, patient care, adverse event reporting (a CRA simply audits and ensures that no AEs were missed!), preparing the case report form (CRF) for the CRA to review against medical records, and submitting tons of data and records to the CRA/Sponsor at each site visit.
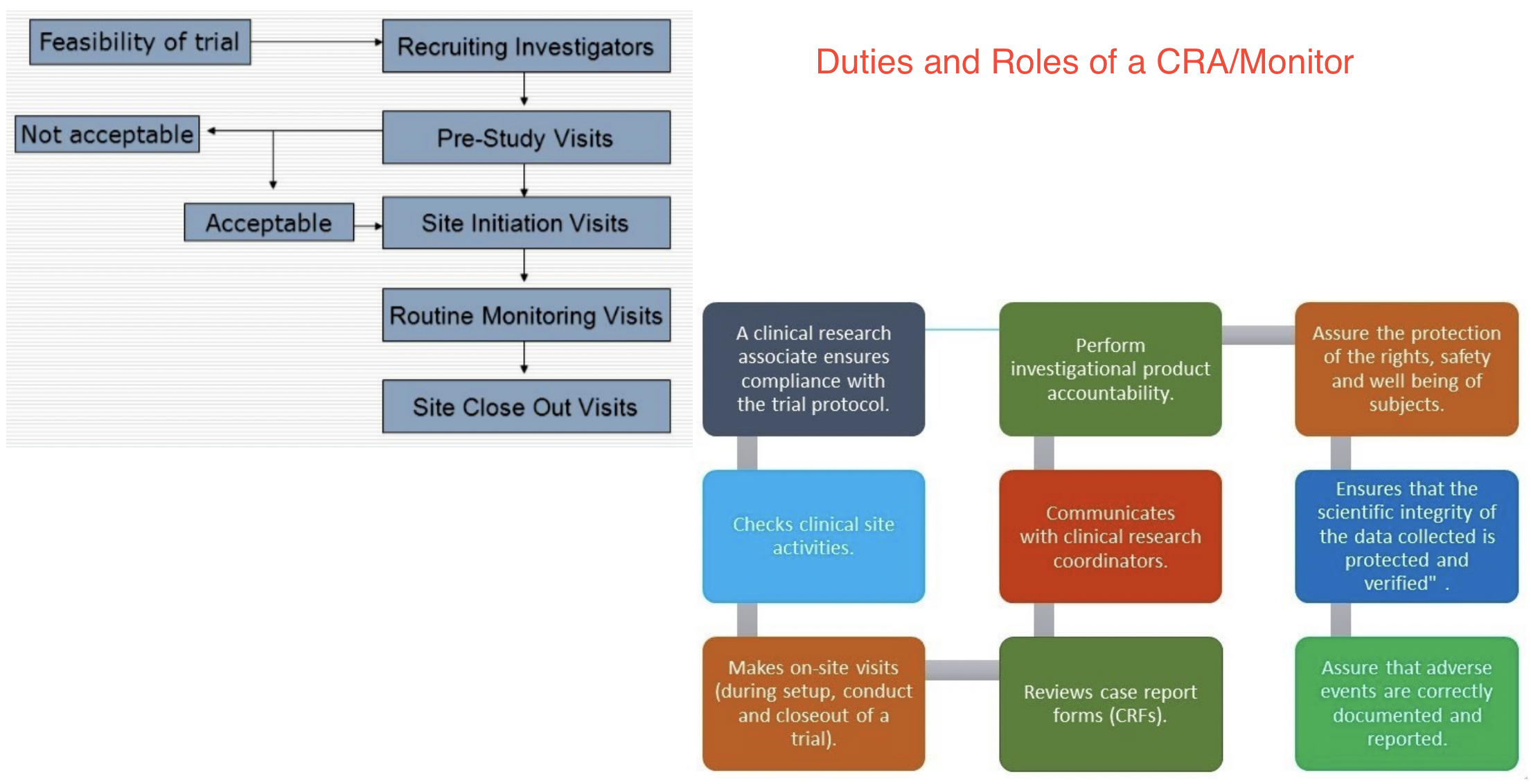
Roles of a CRA
CRA interactions with other fields

Roles of a CRC
CRC interactions with other fields
Clinical Research Associate vs Coordinator Salary
Because CRAs manage multiple trial sites at one time, have a bachelors degree (minimum), and produce outcomes that are cost-effective for improving the efficiency of a trial; clinical research associates usually get paid more than coordinators. Unfortunately, clinical research coordinators are really doing the brunt of the “front-line” work and are the reason the trial occurs at that site all together. CRCs take a huge responsibility in both starting the trial and then presenting the trial documents to the CRAs as well as being the “middle-man” of the entire Site vs. Sponsor/CRO communication line. While CRCs deserve to get paid more, of course, it is not cost-effective as there are usually multiple sites and thus budgets are not capable of expanding upon the CRC’s pay-range. Luckily, CRC’s with experience can bridge to becoming a CRA through certifications and exams.
List of Relevant Courses:
Clinical Research Coordinator Certification: Enroll Here
Pharmacovigilance Certification: Enroll Here
Clinical Research Associate (CRA) Certification: Enroll Here
ICH-GCP Certification: Enroll Here
Clinical Trials Assistant Training: Enroll Here
Advanced Clinical Research Project Manager Certification: Enroll Here
Advanced Principal Investigator Physician Certification: Enroll Here
Medical Monitor Certification: Enroll Here
CRC to CRA bridge program
You can bridge into being a CRA in your own company or apply for jobs to be a CRA by completing CRA certification and trying to get experience with any on-site “in house” CRAs your site may have. CCRPS provides advanced “senior”-level CRA certification for CRCs so that:
1) on resumes you can prove knowledge competency of CRA tasks up to an advanced level (easier for in-job promotion)
2) during interviews you can prove your application of knowledge
3) during the job itself you can be efficient and diligent in preventing errors.
Clinical Research Coordinator Salary
Clinical research associate salary - what's the pay for a clinic research associate.
CRA vs CRC: Demystifying the Roles in Clinical Research
In the world of clinical research, two essential roles take center stage: the Clinical Research Associate (CRA) and the Clinical Research Coordinator (CRC). These professionals play pivotal roles in ensuring the smooth running of clinical trials and the safety and welfare of participants. However, their responsibilities, qualifications, and skills differ. In this article, we will delve into the distinct functions and requirements of CRAs and CRCs, shedding light on their contributions to the field of clinical research.
Understanding the Role of a Clinical Research Coordinator (CRC)
A Clinical Research Coordinator (CRC) functions as a crucial link between the principal investigator and the research team. Their primary responsibility lies in ensuring the seamless execution of clinical trials. The CRC acts as the main point of contact for participants, playing a vital role in recruiting, screening, consenting, and coordinating their involvement in the study.
One of the key responsibilities of a CRC is participant recruitment. They work closely with the principal investigator to develop effective strategies to attract eligible participants to the study. This may involve reaching out to potential participants through various channels, such as social media, community outreach programs, and healthcare providers. The CRC carefully screens potential participants to ensure they meet the study's inclusion and exclusion criteria.
Once participants are enrolled in the study, the CRC plays a crucial role in obtaining informed consent. They explain the study procedures, risks, and benefits to participants, ensuring they have a clear understanding of what their involvement entails. The CRC addresses any questions or concerns participants may have, ensuring their comfort and confidence in participating in the trial.
Furthermore, the CRC collates and maintains accurate and complete study records, including regulatory documentation and source data verification. This meticulous attention to detail is essential to comply with Good Clinical Practice guidelines and ensure data integrity. They work closely with the research team to ensure that all study procedures are documented accurately and in a timely manner.
In addition to documentation, CRCs also play a vital role in the coordination of participant visits. They schedule appointments, prepare study materials, and ensure that all necessary equipment and supplies are available. During participant visits, the CRC may administer investigational products, collect samples, and monitor adverse events. Their commitment to participant safety and the accurate documentation of all trial-related activities is paramount in maintaining the integrity of the study.
Qualifications and Skills Required for a CRC Position
To excel as a Clinical Research Coordinator, certain qualifications and skills are necessary. A minimum of a bachelor's degree in a relevant field is typically required, although some positions may necessitate a more advanced degree. In terms of skills, attention to detail, excellent organizational abilities, and strong interpersonal and communication skills are crucial.
Attention to detail is vital for CRCs as they need to ensure that all study procedures are carried out accurately and in accordance with the protocol. They must be able to spot any discrepancies or errors in the data and take appropriate actions to rectify them. Excellent organizational abilities are also essential as CRCs are responsible for managing multiple tasks simultaneously, such as participant recruitment, scheduling visits, and maintaining study records.
Strong interpersonal and communication skills are crucial for CRCs as they interact with participants, healthcare professionals, and other members of the research team on a regular basis. They need to be able to effectively communicate complex information in a clear and concise manner, ensuring that everyone involved in the study understands their roles and responsibilities.
Additionally, CRCs must possess a solid understanding of study protocols and applicable regulations, such as the International Conference on Harmonisation (ICH) guidelines. They need to stay updated with any changes or updates to these guidelines to ensure that the study is conducted in compliance with the latest standards. Proficiency in data management and familiarity with electronic data capture systems are also highly valued skills in their arsenal. They need to be able to efficiently collect, organize, and analyze data to ensure accurate reporting and analysis of study results.
Demystifying the Role of a Clinical Research Associate (CRA)
When it comes to the world of clinical research, there are many important roles that contribute to the success of a study. One such role is that of a Clinical Research Associate (CRA). While the duties and responsibilities of a CRA may not be as well-known as those of a Clinical Research Coordinator (CRC), they are just as crucial to the smooth operation of clinical trials.
Unlike CRCs who primarily focus on the day-to-day management of clinical trials, CRAs specialize in monitoring the progress of these trials. They play a vital role in ensuring that clinical sites adhere to the study protocol, regulatory requirements, and ethical guidelines. This involves conducting routine site visits to assess data quality, participant safety, and adherence to Good Clinical Practice (GCP) standards.
One of the key responsibilities of a CRA is to review and verify study data and source documents. This meticulous analysis of trial data is essential to ensure its accuracy and alignment with the protocol. CRAs also play a crucial role in ensuring that the data meets regulatory requirements. Their objective viewpoint allows them to identify and resolve any discrepancies or potential issues at clinical sites, contributing to the overall success of the study.
Essential Skills and Qualifications for a Successful CRA Career
Embarking on a successful career as a Clinical Research Associate requires a combination of technical expertise and interpersonal skills. While a bachelor's degree in a life science discipline or a related field is typically required for entry-level positions, advanced degrees such as a master's or a Ph.D. can enhance career prospects.
Attention to detail is one of the most important skills for a CRA. They must have a keen eye for spotting any inconsistencies or errors in the data they review. Strong analytical and problem-solving skills are also essential, as CRAs often encounter complex issues that require quick and effective solutions.
A comprehensive understanding of clinical trial processes is another prerequisite for CRAs. They must be well-versed in the intricacies of study protocols, regulatory guidelines, and GCP standards. Additionally, proficiency in reviewing and verifying clinical data is highly valued. Experience with electronic data capture systems is also beneficial, as many clinical trials now rely on these systems for data collection and management.
Effective communication and relationship-building abilities are crucial for CRAs. They must be able to coordinate effectively with investigators, site staff, and sponsors to ensure the smooth running of the study. Building strong relationships with these stakeholders is essential for maintaining open lines of communication and resolving any issues that may arise.
In conclusion, the role of a Clinical Research Associate is multifaceted and plays a vital role in the success of clinical trials. Their responsibilities encompass monitoring the progress of trials, ensuring compliance with protocols and regulations, and reviewing and verifying study data. To excel in this role, CRAs must possess a combination of technical expertise, attention to detail, problem-solving skills, and effective communication abilities.
Exploring the Overlapping Responsibilities of a CRA and a CRC
Common tasks and responsibilities shared by cras and crcs.
Although the roles of CRAs and CRCs differ, they share several common tasks and responsibilities. Both CRAs and CRCs contribute to the recruitment and retention of study participants, ensuring compliance with protocols and regulatory requirements.
Moreover, both roles involve meticulous documentation and record-keeping. CRAs and CRCs must maintain accurate, complete, and organized study records to enable data verification and auditing. This shared commitment to data integrity and participant welfare underscores the vital importance of collaboration between the two roles.
Collaboration and Communication: Key Aspects of Both Roles
Effective collaboration and communication are critical components of both the CRA and CRC roles. CRAs must establish strong working relationships with investigators, site staff, and sponsors, facilitating open lines of communication to address challenges and drive study progress. Similarly, CRCs must communicate effectively with participants, ensuring informed consent, conveying study-specific information, and addressing any concerns or questions.
Collaboration between CRAs and CRCs is crucial during site visits, as CRAs rely on the expertise and support of CRCs to facilitate data collection, sample handling, and adverse event reporting. This collaborative approach promotes synergy and fosters a positive research environment, ultimately benefiting the participants and overall study outcomes.
Key Distinctions Between a CRA and a CRC
Understanding the unique responsibilities of a cra.
While CRAs and CRCs share certain responsibilities, it is essential to grasp the unique aspects of each role. CRAs primarily focus on monitoring and ensuring compliance with study protocols, analyzing data for accuracy, and identifying and resolving issues at clinical sites.
CRAs act as the liaison between sponsors, sites, and investigators, facilitating smooth communication and driving study progress. Their expertise in interpreting and applying regulations and guidelines contributes to effective clinical trial oversight.
Differentiating the Role of a CRC in Clinical Research
In contrast, CRCs perform vital tasks related to participant interaction and coordination. Their responsibilities encompass recruiting and screening individuals for study eligibility, collecting specimens, and maintaining accurate records.
CRCs ensure proper participant consent, adherence to protocols, and the overall delivery of quality data. Their meticulous approach and attention to detail contribute significantly to the successful implementation of clinical trials.
Wrapping Up: Comparing CRAs and CRCs in Clinical Research
In summary, the roles of the Clinical Research Associate (CRA) and the Clinical Research Coordinator (CRC) are distinct yet interconnected. CRAs focus on monitoring and ensuring compliance, while CRCs are responsible for participant coordination and data management.
Both roles play critical roles in the successful execution of clinical trials, serving as pillars of ethical research. By understanding the unique responsibilities, qualifications, and skills of CRAs and CRCs, we can appreciate the symbiotic relationship that drives advancements in clinical research for the betterment of global health .
If you're inspired by the critical roles that CRAs and CRCs play in the advancement of clinical research and are looking for a comprehensive solution to streamline your clinical trials, Lindus Health is here to support you. As a full-service CRO, we offer a complete stack of services to manage your clinical trial from start to finish. Our all-in-one eClinical platform, coupled with expert site services, ensures your study is executed with precision and efficiency. Don't hesitate to book a meeting with our team today and discover how we can elevate your clinical research endeavors.

Download now
Related stories, speak with an expert about your study..

What can Lindus Health do for your study?
Quick links, your trial, solved..

Research Assistant vs. Research Coordinator: What Are the Differences?
Learn about the two careers and review some of the similarities and differences between them.
If you’re interested in a career in research, you may be wondering what the difference is between a research assistant and a research coordinator. Both roles are important in the research process, but they have different responsibilities. In this article, we compare and contrast the job duties of research assistants and research coordinators, and we provide information on education and skills requirements.
What is a Research Assistant?
A research assistant is a professional who supports the work of scientists, typically in academic or industrial research laboratories. They may be involved in a range of activities, from conducting experiments to maintaining equipment to documenting results. Many research assistants are undergraduate or graduate students who are working towards a degree in a scientific field, although some may be experienced professionals. In some cases, research assistants may be responsible for leading a project or team of researchers.
What is a Research Coordinator?
Research Coordinators work in academic, government and private industry settings to manage research projects from start to finish. They develop research proposals, secure funding and ethical approval, recruit and manage research staff, collect and analyze data, write reports and papers, and present findings to clients or other interested parties. Research Coordinators typically have a background in research methods and statistics, and they use their skills to design and implement studies that produce valid and reliable results. They must be able to effectively communicate with research staff, clients and other stakeholders to ensure that all parties are kept up to date on the progress of the project and that the final product meets the needs of the client.
Research Assistant vs. Research Coordinator
Here are the main differences between a research assistant and a research coordinator.
Research assistants perform more hands-on tasks and research coordinators handle more administrative responsibilities. For example, a research assistant might interview study participants, administer questionnaires or conduct laboratory tests. They also analyze the data and write reports based on their findings.
Research coordinators may schedule research assistants’ appointments, provide them with support and communicate with them about project details. They also maintain research records, such as test results and previous research papers. Additionally, research coordinators may help researchers develop research proposals and manage budgets.
Job Requirements
Research assistants and research coordinators typically need a bachelor’s degree in a scientific field, such as biology, chemistry or physics. Some employers may prefer candidates with a master’s degree or higher. Additionally, research assistants and research coordinators might need to have experience working in a laboratory setting. Many professionals in these roles start out as lab technicians before moving into more senior positions.
Work Environment
Research assistants typically work in a variety of environments, depending on the project they’re working on. They may spend time in libraries or other research facilities to find information for their projects. Some research assistants also work directly with clients and collaborate with them to determine what information is needed for their projects.
Research coordinators usually work in an office environment where they manage all aspects of their department’s operations. They often have regular hours and perform administrative tasks like scheduling meetings and managing budgets.
Both research assistants and research coordinators need to have excellent research skills. This includes the ability to find relevant information, critically evaluate sources and synthesize data. They also both need to be able to communicate effectively, whether it is writing reports or presenting findings to colleagues.
However, research coordinators tend to have more responsibilities than research assistants. In addition to conducting research, they may also be responsible for managing projects, supervising other research staff and coordinating with different teams. This can require additional skills, such as project management, team leadership and budgeting.
Research assistants earn an average salary of $42,572 per year, while research coordinators earn an average salary of $62,030 per year. The average salary for both positions may vary depending on the type of research you’re conducting, the size of the company you work for and the level of experience you have in the field.
Boilermaker vs. pipefitter: What Are the Differences?
Patient care coordinator vs. receptionist: what are the differences, you may also be interested in..., screenwriter vs. director: what are the differences, lawyer vs. teacher: what are the differences, controller vs. finance manager: what are the differences, test analyst vs. test engineer: what are the differences.
Research Assistant vs. Research Coordinator – What’s The Difference?

- Updated February 20, 2023
- Published February 13, 2023
Research Assistant vs. Research Coordinator – what are the differences? Learn everything you need to know about the differences between a Research Assistant and a Research Coordinator.
Research Assistants and Research Coordinators are both important roles in the research field. However, there are distinct differences between the two.
For example, Research Assistants are typically responsible for tasks such as collecting and organizing data, conducting literature reviews, and running statistical analyses. Research Coordinators, on the other hand, are usually responsible for designing and managing research projects, developing protocols, and overseeing the implementation of research protocols.
What is a Research Assistant?
A research assistant is an individual who provides administrative, technical, and research support to a research team. Research assistants may be responsible for conducting literature reviews, data entry, data analysis, and other tasks to support the research process.
Research assistants may work in a variety of fields, such as academia, government, and private industry.
What is a Research Coordinator?
A Research Coordinator is a professional who assists with the planning, implementation, and management of research projects. They work with researchers to develop objectives, timelines, and budgets, and they may be responsible for recruiting and managing research participants, collecting and analyzing data, and writing reports.
Research Coordinators may also provide guidance and advice on research methodology and design.
Research Assistant vs. Research Coordinator
Below we discuss the fundamental differences between work duties, work requirements, and work environment of a Research Assistant and a Research Coordinator.

Research Assistant vs. Research Coordinator Job Duties
When it comes to educational and job experience, there are distinct differences between the roles of a research assistant and a research coordinator. Both positions can be found in academic, medical, and corporate research settings and require very specific skill sets.
A research assistant is typically responsible for a range of tasks related to data collection and analysis, literature reviews, and other support tasks. Research assistants typically require a bachelor’s degree in a field related to the research project, such as psychology, sociology, biology, or economics. They should have strong analytical and research skills, be proficient in data collection, and have experience with Microsoft Office applications.
By contrast, research coordinators are typically responsible for the overall management of research projects. They are expected to have a higher level of experience and knowledge, such as a master’s degree in a field related to the research project, and should have experience in management, budgeting, and project planning.
Research coordinators are also responsible for communicating and coordinating between the research team and other stakeholders, ensuring that deadlines are met and research results are properly documented.
In summary, research assistants are typically responsible for collecting and analyzing data and literature reviews, while research coordinators are responsible for the overall research project management.
Both positions require a degree in a field related to the research project and experience in the respective area. However, research coordinators typically require a higher level of experience and knowledge and are expected to have a master’s degree in a related field.
Related : Associate Scientist vs. Research Associate: What’s The Difference?
Research Assistant vs. Research Coordinator Job Requirements
Research assistants and research coordinators are both professionals in the field of research, but they have different job requirements, including education.
In general, research assistants are entry-level professionals who work under the guidance of more experienced researchers, while research coordinators have more experience and are responsible for managing research projects. Here are the specific differences in job requirements between research assistants and research coordinators:
- Research assistants typically need a bachelor’s degree in a field related to the research they will be assisting with, such as biology, psychology, or sociology. Some employers may require a master’s degree for more complex research projects.
- Research coordinators usually need a master’s degree in a related field, such as public health or clinical research, and may also be required to have research experience.
Experience:
- Research assistants typically do not require any previous research experience, although it can be helpful. They are usually responsible for tasks such as collecting data, performing literature searches and preparing materials for research studies.
- Research coordinators are more experienced professionals who have a background in research and typically have at least 3-5 years of experience. They are responsible for managing research projects, including coordinating with other team members, monitoring study progress, and ensuring compliance with ethical standards.
Job responsibilities:
- Research assistants may perform tasks such as collecting data, recruiting participants, preparing research materials, and assisting with data analysis.
- Research coordinators may be responsible for overseeing research studies from start to finish, including creating research protocols, recruiting, and training staff, coordinating study logistics, monitoring data quality, and ensuring compliance with regulatory requirements.
Overall, while both research assistants and research coordinators play an important role in the research process, research coordinators have a more advanced level of responsibility and require a higher level of education and experience.
Research Assistant vs. Research Coordinator Work Environment
Research assistants and research coordinators work in research settings, such as academic institutions, government agencies, non-profit organizations, and private companies, to support research studies and projects. While there may be some overlap in their work environments, there are also some notable differences in the work environments of these two roles.
Research assistants typically work under the direct supervision of a principal investigator or senior researcher. They may work as part of a team or may work independently on smaller projects. Research assistants may be required to work in laboratory settings, conducting experiments or collecting data, or may work in office settings, analyzing data and conducting literature reviews.
In general, research assistants work on the ground level of a research project, executing the tasks assigned to them by the senior researchers. Their work may involve operating laboratory equipment, gathering and analyzing data, and participating in the preparation of research findings for publication. The work environment for research assistants can be fast-paced and dynamic and may require the ability to work on multiple tasks simultaneously.
On the other hand, research coordinators typically work in more administrative roles, overseeing and coordinating the various aspects of a research study. Research coordinators are responsible for organizing the logistics of the study, ensuring that it meets regulatory requirements and stays on schedule. They may also be responsible for recruiting and managing study participants, managing the study budget, and communicating with other research professionals, such as data analysts and statisticians.
Research coordinators may work in both laboratory and office settings but typically spend more time in an office environment. They may work closely with study participants, managing communication and scheduling appointments, as well as with other research professionals to ensure the study runs smoothly.
Overall, the work environment for research coordinators tends to be more structured and administrative, while the work environment for research assistants tends to be more dynamic and hands-on.
Both roles require a strong attention to detail and strong communication and organizational skills, but the specific nature of the work requires different strengths and expertise.
Research Assistant vs. Research Coordinator Skills
Here is a comparison of the required job skills between a Research Assistant and a Research Coordinator:
Research Assistant:
- Conducting experiments: Research assistants must have strong lab skills and be able to accurately and safely conduct experiments according to the protocols and guidelines provided to them.
- Data collection and analysis: Research assistants collect, organize, and analyze data from experiments. They should be proficient in relevant software and statistical methods.
- Communication skills: Research assistants must be able to communicate effectively with other team members and accurately report on their findings.
- Attention to detail: They should possess keen attention to detail to ensure experiments are performed correctly, and data is accurately collected.
Research Coordinator:
- Project management skills: Research coordinators oversee multiple research projects and, as such, must have strong project management skills, including the ability to prioritize and manage timelines, delegate tasks, and meet deadlines.
- Regulatory compliance: Research coordinators must be familiar with research regulations and guidelines, such as the Health Insurance Portability and Accountability Act (HIPAA), and ensure that all research activities adhere to them.
- Data management: They should possess strong data management skills and be proficient in relevant software used for collecting, organizing, and analyzing data.
- Supervisory skills: Research coordinators must have the ability to manage and supervise research assistants and other staff members.
- Communication skills: They should be able to communicate effectively with researchers, research subjects, and other team members to ensure the smooth operation of research activities.
In summary, while both Research Assistants and Research Coordinators require proficiency in data management and communication skills, Research Coordinators also need project management and supervisory skills and regulatory compliance knowledge.
On the other hand, research assistants require a focus on lab skills and attention to detail.
Research Assistant vs. Research Coordinator Salary
When considering a career in research, it is important to consider the various roles available and the corresponding pay. Research Assistants and Research Coordinators are two of the most common positions in research, and the pay difference between these roles can be significant.
Research Assistants primarily assist with conducting research studies, collecting and analyzing data, and writing reports. They typically work under the supervision of a Research Coordinator. Their primary duties include researching topics, preparing materials, collecting and entering data, preparing reports, and assisting in designing experiments.
Research Assistants are typically paid hourly or per-project, and the amount of money they earn depends on the specific research project, their qualifications, and the amount of time they put into the project.
Research Coordinators, on the other hand, are typically overseeing the entire research process. This includes working with researchers to design experiments, collecting and analyzing data, writing reports, and managing the budget for the research project.
Research Coordinators are typically paid a salary, and the amount of money they make depends on their experience, qualifications, and the specific research project.
Overall, Research Assistants will typically make less money than Research Coordinators. Research Assistants typically make between $15-$25 an hour, while Research Coordinators typically make between $35,000-$50,000 a year.
When deciding between the two roles, it is important to consider the qualifications and experience required for each role. Research Assistants typically require a Bachelor’s degree in a relevant field, while Research Coordinators usually require a Master’s degree in a relevant field.
Additionally, Research Coordinators are typically expected to have more experience and knowledge in research methods and techniques than Research Assistants.
Ultimately, both roles offer rewarding and interesting work as part of a research team. The amount of money earned will largely depend on the specific research project and the individual’s qualifications and experience.
Related posts:
- Clinical Research Coordinator Cover Letter Examples & Writing Guide
- Clinical Research Coordinator Interview Questions & Answers
- Administrative Coordinator vs. Administrative Assistant: What’s The Difference?
- Office Coordinator vs. Administrative Assistant – What’s The Difference?
- Associate Scientist vs. Research Associate: What’s The Difference?
Rate this article
Your page rank:
MegaInterview Company Career Coach
Step into the world of Megainterview.com, where our dedicated team of career experts, job interview trainers, and seasoned career coaches collaborates to empower individuals on their professional journeys. With decades of combined experience across diverse HR fields, our team is committed to fostering positive and impactful career development.
You may also be interested in:
Corporate controller vs controller – what’s the difference, detention officer vs. correctional officer – what’s the difference, loan processor vs. underwriter – what’s the difference, supervising producer vs. executive producer – what’s the difference, interview categories.
- Interview Questions
- Cover Letter
- Interview Tips
Megainterview/Contact
- Career Interview Questions
- Write For Megainterview!
- Editorial Policy
- Privacy Policy / GDPR
- Terms & Conditions
- Contact: [email protected]
Sign-up for our newsletter
🤝 We’ll never spam you or sell your data
Popular Topics
- Accomplishments
- Adaptability
- Career Change
- Career Goals
- Communication
- Conflict Resolution
- Creative Thinking
- Critical Thinking
- Cultural Fit
- Customer Service
- Entry-Level & No Experience
- Growth Potential
- Honesty & Integrity
- Job Satisfaction
- Negotiation Skills
- Performance Based
- Phone Interview
- Problem-Solving
- Questions to Ask the Interviewer
- Salary & Benefits
- Situational & Scenario-Based
- Stress Management
- Time Management & Prioritization
- Uncomfortable
- Work Experience
Popular Articles
- What Is The Most Challenging Project You Have Worked On?
- Tell Me About a Time You Had to Deal With a Difficult Customer
- What Have You Done To Improve Yourself In The Past Year?
- Interview Question: How Do You Deal With Tight Deadlines?
- Describe a Time You Demonstrated Leadership
- Tell Me About a Time When You Took Action to Resolve a Problem
- Job Interview Questions About Working in Fast-Paced Environments
- Job Interview: What Areas Need Improvement? (+ Answers)
- Tell Me About a Time You Were On a Team Project That Failed
- Tell Me About a Time You Managed an Important Project
Our mission is to
Help you get hired.
Hofplein 20
3032 AC, Rotterdam, the Netherlands
Turn interviews into offers
Every other Tuesday, get our Chief Coach’s best job-seeking and interviewing tips to land your dream job. 5-minute read.
Explore Jobs
- Jobs Near Me
- Remote Jobs
- Full Time Jobs
- Part Time Jobs
- Entry Level Jobs
- Work From Home Jobs
Find Specific Jobs
- $15 Per Hour Jobs
- $20 Per Hour Jobs
- Hiring Immediately Jobs
- High School Jobs
- H1b Visa Jobs
Explore Careers
- Business And Financial
- Architecture And Engineering
- Computer And Mathematical
Explore Professions
- What They Do
- Certifications
- Demographics
Best Companies
- Health Care
- Fortune 500
Explore Companies
- CEO And Executies
- Resume Builder
- Career Advice
- Explore Majors
- Questions And Answers
- Interview Questions
Clinical Research Assistant Vs Clinical Research Coordinator
The differences between clinical research assistants and clinical research coordinators can be seen in a few details. Each job has different responsibilities and duties. It typically takes 1-2 years to become both a clinical research assistant and a clinical research coordinator. Additionally, a clinical research coordinator has an average salary of $52,459, which is higher than the $39,837 average annual salary of a clinical research assistant.
The top three skills for a clinical research assistant include patients, informed consent and data collection. The most important skills for a clinical research coordinator are patients, informed consent, and IRB.
Clinical research assistant vs clinical research coordinator overview
What does a clinical research assistant do.
Clinical research assistants are responsible for assisting with scientific studies and monitoring clinical trials. Other duties and responsibilities include ensuring compliance with clinical trial procedures and protocols, finding research subjects, and collecting and analyzing data. In addition, they are responsible for overseeing clinical site activities and preparing documentation, presentation, and correspondence on findings. They are also expected to prepare informed consent for clinical trials and conduct audits on research trials. The skills and qualifications required for this role include a bachelor's degree in psychology, science or related field, previous work experience, and excellent communication skills .
What does a clinical research coordinator do?
A clinical research coordinator is a healthcare professional responsible for administering clinical trials of drugs or medications. Clinical research coordinators work under the supervision of clinical research managers to collect data and help inform trial participants about the study's objectives. They must ensure that these trials have met all regulations, including drug safety, government regulations, and the organization's code of ethics. Clinical research coordinators must obtain a bachelor's degree in nursing and have at least two years of healthcare experience.
Clinical research assistant vs clinical research coordinator salary
Clinical research assistants and clinical research coordinators have different pay scales, as shown below.
Differences between clinical research assistant and clinical research coordinator education
There are a few differences between a clinical research assistant and a clinical research coordinator in terms of educational background:
Clinical research assistant vs clinical research coordinator demographics
Here are the differences between clinical research assistants' and clinical research coordinators' demographics:
Differences between clinical research assistant and clinical research coordinator duties and responsibilities
Clinical research assistant example responsibilities..
- Manage patient caseload with emphasis in occupational rehabilitation
- Maintain compliance with ICH, GCP and federal regulations as well as reporting adverse events to the FDA.
- Perform medical assessment procedures including phlebotomy, laboratory processing, vital signs, and electrocardiogram.
- Skil in CPR and patient distress.
- Transport patients, and restive beds from ICU and IMCU units
- Specialize training such as, CPI non-violent crisis intervention and CPR.
Clinical Research Coordinator Example Responsibilities.
- Assess study participants for adverse reactions or complications and manage side effects of chemotherapy and other study relate drugs.
- Establish and maintain strong community partnerships to achieve HIV prevention objectives.
- Direct acquisition and analysis of functional MRI research data following GCP and FDA regulations
- Prepare and maintain IRB and regulatory documentation for various research projects and consent patients for clinical research.
- Facilitate constant communication between principal investigators, oncology nurses, and patients to address concerns and maximize eligibility and enrollment.
- Schedule patient for study procedures; initiate/coordinate drug orders, laboratory procedures and treatments for patients base on standing protocol orders.
Clinical research assistant vs clinical research coordinator skills
- Patients, 16%
- Informed Consent, 8%
- Data Collection, 7%
- Data Entry, 6%
- Patient Care, 4%
- Patients, 11%
- Informed Consent, 10%
- Data Collection, 5%
- Research Projects, 4%
Clinical Research Assistant vs. Similar Jobs
- Clinical Research Assistant vs Associate
- Clinical Research Assistant vs Coordinator
- Clinical Research Assistant vs Research Assistant
- Clinical Research Assistant vs Clinical Research Associate
- Clinical Research Assistant vs Clinical Researcher
- Clinical Research Assistant vs Clinical Trial Coordinator
- Clinical Research Assistant vs Clinical Assistant
- Clinical Research Assistant vs Clinical Research Coordinator
- Clinical Research Assistant vs Clinical Research Nurse
- Clinical Research Assistant vs Research Nurse
- Clinical Research Assistant vs Senior Clinical Research Associate
- Clinical Research Assistant vs Research Coordinator
- Clinical Research Assistant vs Research Project Coordinator
- Clinical Research Assistant vs Study Coordinator
- Clinical Research Assistant vs Senior Research Associate
Clinical Research Assistant Related Careers
- Clinical Assistant
- Clinical Associate
- Clinical Coordinator
- Clinical Project Manager
- Clinical Research Associate
- Clinical Research Coordinator
- Clinical Research Manager
- Clinical Research Nurse
- Clinical Trial Manager
- Coordinator And Research Assistant
- Research Administrator
- Research Assistant
- Research Coordinator
- Research Nurse
- Research Project Coordinator
Clinical Research Assistant Related Jobs
- Clinical Assistant Employment Near Me
- Clinical Associate Employment Near Me
- Clinical Coordinator Employment Near Me
- Clinical Project Manager Employment Near Me
- Clinical Research Associate Employment Near Me
- Clinical Research Coordinator Employment Near Me
- Clinical Research Manager Employment Near Me
- Clinical Research Nurse Employment Near Me
- Clinical Trial Manager Employment Near Me
- Coordinator And Research Assistant Employment Near Me
- Research Administrator Employment Near Me
- Research Assistant Employment Near Me
- Research Coordinator Employment Near Me
- Research Nurse Employment Near Me
- Research Project Coordinator Employment Near Me
What Similar Roles Do
- Clinical Assistant Responsibilities
- Clinical Associate Responsibilities
- Clinical Coordinator Responsibilities
- Clinical Project Manager Responsibilities
- Clinical Research Associate Responsibilities
- Clinical Research Coordinator Responsibilities
- Clinical Research Manager Responsibilities
- Clinical Research Nurse Responsibilities
- Clinical Trial Manager Responsibilities
- Coordinator And Research Assistant Responsibilities
- Research Administrator Responsibilities
- Research Assistant Responsibilities
- Research Coordinator Responsibilities
- Research Nurse Responsibilities
- Research Project Coordinator Responsibilities
- Zippia Careers
- Executive Management Industry
- Clinical Research Assistant
Browse executive management jobs
Career Progression in Clinical Research: Transitioning from a Clinical Research Coordinator to a Monitoring Clinical Research Associate (CRA)

Thomas Boothby, MS, CCRP CRA II, Boston Scientific
Abstract : Research coordinators may transition to clinical research associates/monitors during their careers. This article provides an overview of how to determine whether it is the right time to make this transition, how to evaluate current competencies and gaps that must be filled in order to make this transition, and how to address needs during the on-boarding process. A roadmap in the form of a checklist is provided to help make the transition from research coordinator to clinical research associate (CRA) a smooth one.
Disclosure: The author has a relevant financial relationship with respect to this article with Boston Scientific, where he is employed as a monitoring CRA.
Introduction
A research coordinator is a person at the clinical research site who is involved in the daily tasks of enrollment, data entry, and all other aspects of clinical trials at the site level. A clinical research associate (CRA), or monitor, is the individual who visits clinical research sites to review their medical records and do the standard monitoring visits. Before the author was a CRA, he was a research coordinator for fourteen years. This article describes how the author made the transition from clinical research coordinator to CRA/clinical research monitor and includes some suggestions for those looking to make a similar career change.
When to Transition from Research Coordinator to CRA
While people naturally want to progress their careers as fast as possible, it is important to only make thetransition from research coordinator to CRA when the time is right. The grass is not always greener on the other side of the work fence.
The author knew that he was ready to make the transition from research coordinator to CRA because he felt that he had mastered all the tasks of a research coordinator. His job became stagnant, and he was looking for something better. Fatigue in the current work environment is another reason for why individuals may be looking to make this transition. Of all members of the clinical research team, research coordinators have the most difficult job. In the author’s opinion, they are often overworked and underpaid, and their contributions to the overall study are sometimes overlooked. Other reasons to make the transition from research coordinator to CRA include potential career progression and the opportunity to try something new. Some individuals may find that the travel component that goes along with being a monitor is a positive as well.
There are five stages of change according to a behavioral change model: pre-contemplation, contemplation, determination, action, and maintenance. In the pre-contemplation phase, people are not thinking about transitioning yet or may have obstacles in their daily lives that are preventing them from exploring new opportunities. When people are becoming serious about change, they are in the determination or action phases. During these phases, research coordinators who want to transition to CRAs might apply for new positions or become certified clinical research professionals (SOCRA CCRP®) as they try to gain new skills for the job market. When considering a transition from research coordinator to CRA, it is important to identify one’s place in the behavioral change model.
Qualifications and Background of CRAs
When the author was applying for CRA positions in 2015, he always saw a requirement for at least two years of experience as a monitor. This requirement is often a barrier to those looking to make this career transition. In 2010, ClinicalTrials.gov listed more than 100,000 clinical studies. By 2019, that number has increased to more than 300,000 clinical studies. The clinical research market has exploded over the last decade. More people are needed to monitor and to run clinical studies now than ever before. While some companies are less likely to require two years of monitoring experience now due to a depleted pool of candidates, these same companies may be more open to supplemental forms of experience such as certifications, course work, and on-the-job experience.
Thus, this is a great time to act on the decision to transition from research coordinator to CRA. From 2014 to 2024, the United States Bureau of Labor Statistics estimates that CRA positions will increase 14% annually. This increase in the job market, coupled with the high level of CRA turnover, could lead to a very strong job market in the future. At Boston Scientific, turnover among CRAs is fairly low due to the strong structure and principles. Many CRAs within Boston Scientific have been with the company for 10 to 20 years or longer.
Table 1 highlights the typical background of CRAs. Most CRAs are current or former nurses who have experience as a research coordinator or a research assistant. Many universities now offer bachelor’s, master’s, and certificate programs in clinical research as another form of training for these research related roles. In Michigan, where the author is from, Eastern Michigan University has a two-year master’s degree program in clinical research. Like the author, CRAs can often be a former research coordinator.
When the author was transitioning from research coordinator to CRA, he got his foot in the door by working closely with a monitor who still works for Boston Scientific. Relationships between research coordinators and CRAs can be contentious due to the nature of monitoring. Research coordinators should treat monitors and sponsor staff well and with respect, and they should treat monitoring visits as a learning opportunity and not a criticism of the coordinator’s work. These relationships do not need to be contentious. A good working relationship with a clinical research site’s CRAs can serve as a potential audition for a monitoring position.
CRAs typically have a clinical research certification, either SOCRA’s certified clinical research professional (CCRP®) or the Association of Clinical Research Professionals-Certified Professional (ACRP-CP). Some companies provide tuition reimbursement for programs and certifications such as these as a way of employee enhancement. Research coordinators can participate in enrichment programs such as these and obtain certifications to help boost their resume and become more marketable to CROs and sponsors. When researching these programs, individuals must do their due diligence to ensure that the program or certification is offered by a legitimate organization and is accredited. Hiring managers know where to find the gold standards in clinical research programs and certifications, and those that do not fit this standard can even be viewed as a negative on ones resume.
The author is a SOCRA CCRP®, Certified Clinical Research Professional, which is an excellent indicator of knowledge for a monitoring position. The test includes knowledge of the regulations and the role of the monitor. There are also some CRO-development programs such as SOCRA’s Clinical Research Monitoring Conference and one-year certificate programs such as the Harvard Medical School global clinical scholar’s research training program.
Networking through the clinical research site’s CRAs and professional forums and groups such as SOCRA is a great way to find CRA positions and interact with other research professionals. At conferences, CROs often have booths in the exhibit hall where research coordinators can meet CRO staff, learn more about opportunities, and leave their resume with CRO staff.
A Typical Day in the Life of a CRA
The life of a CRA has its positives and negatives (Table 2). There are many things that the author wishes he knew before he became a monitor. The author works from home a great deal of the time. If he is not on the road visiting a clinical research site, he is working at home either preparing for a visit, writing follow-up visit letters, or performing other administrative work. Visit preparation and follow up is a crucial part of the home office work. CRAs have very strict compliance guidelines for completing monitoring visits and monitoring reports in a timely manner. Since recently becoming a lead CRA, the author has also been doing a great deal of administrative and compliance work with more of a global view of a clinical trial.
Some clinical research organizations (CROs) and sponsors have onsite monitors who can do remote visits and activities. Whether visits are onsite or remote, monitors are constantly in contact with clinical research sites to follow up on action items from monitoring visits or to answer protocol specific questions the site coordinators may have.
At most companies, about 60-80% of the monitor’s time is spent traveling to sites. The author currently covers all of Michigan, and he has covered other areas, including Wisconsin, New York, Pennsylvania, and Ohio. CRAs are often away for several days at a time depending on the current workload. This can be difficult on families and personal relationships. While the author travels extensively, there are some times when he travels more than others. Sometimes he does back-to-back visits and may be gone for several days at a time. After that, he may be home for several days. The extensive travel required of CRAs is a key consideration when exploring this career transition.
Being a CRA takes a great deal of self-discipline. Monitoring offers a flexible work arrangement, so monitors can work later in the day or take time off during the normal workday. However, if the CRA does not accomplish what he/she should accomplish, this will be glaringly obvious. Management and co-workers will immediately know if the CRA does not show up to meetings or has difficulty answering questions about his/her monitoring activities or their monitor role in general.
Starting a Monitoring Job
Boston Scientific has a rigorous onboarding process comprised of four to six months of training. After the author was hired as a CRA, he spent months learning the work instructions and going out on preceptor visits. In the beginning, the new CRA observes a senior CRA. Over time, the new CRA does more of the monitoring. By the end of the training, the new CRA is doing the monitoring visit, and the senior CRA is observing and making suggestions to the new hire on how the new hire can improve.
There are various levels of monitors at Boston Scientific: CRA I (for new hires), CRA II, and senior CRA. More experienced CRAs often mentor new CRAs. It is extremely helpful to find CRAs who can serve as mentors and answer questions.
CROs and sponsors have many systems that CRAs must learn. At Boston Scientific, these systems include electronic data capture, clinical trial management, auxiliary programs to help remote employees, and cloud-based filing systems. Being a CRA might be very difficult for people who are resistant to change or have difficulty with technology.
There are several types of monitoring (Table 3). The author would be considered a traditional CRA or monitor. By this, he does traditional onsite monitoring via annual or semi-annual visits to clinical research sites based upon the study’s monitoring plan. At smaller organizations, monitors may travel more often or may have an expanded territory to cover. It is important to ask how much travel is involved and how many monitors are on the team during the interview process. If a company has fewer monitors, more travel will be involved.
Many Boston Scientific protocols require annual monitoring visits. The author visits his clinical research sites at minimum once a year but generally 2-3 times per year. Some of the more difficult sites, high enrollers, and those that are more likely to be inspected by the U.S. Food and Drug Administration are monitored more often. Many sites are participating in more than one Boston Scientific study. For example, the author monitors a site in New York that is conducting several studies. He will monitor two studies during one visit. This saves him time and travel and saves the company money by reducing travel costs. Boston Scientific also uses a risk-based monitoring strategy.
In-house regulatory CRAs at Boston Scientific, called trial management CRAs, interact with the sites on regulatory matters, study startup, and study closure. They work primarily by email and lean on traditional CRAs such as the monitor to be the face of the company with the research coordinators and help ensure that tasks are completed on time. Many hospitals also run their own clinical studies and may have in-house monitors.
Boston Scientific does use remote monitoring in certain studies and circumstances. Remote monitoring takes a great deal of work and technological experience at both the sponsor and site level. It involves a great deal of scanning and correspondence by the research coordinators, which can take a lot of their time and resources.
Sponsor CRAs generally deal with one indication, while CRO CRAs can work on studies for different indications or therapies. In one month, for example, CRO CRAs may be doing four indications at four sites for four sponsors. This requires understanding a great deal of information and being able to use different systems. Good organization is key when working as a CRA, whether for a sponsor or a CRO.
Recently, the author progressed from a CRA II to a senior CRA. As a senior CRA, the author has a larger leadership role and is expected to participate more in training and mentoring other CRAs. Boston Scientific has some centralized monitoring that will look at certain metrics and internal documents to guide monitors in their daily monitoring activities. Monitors are closely linked to the trial managers who actually run the studies. They also deal with safety and data managers as well as their CRA manager and the director of operations. Boston Scientific recently created an associate clinical trial manager position as a way to slowly transition some staff members into clinical trial managers, and the author is also transitioning into this role.
One common drawback about this transition process from research coordinator to CRA is that a CRA is one step removed from patient care. Working directly with patients as a research coordinator is something that the author misses. It is important to remember that CRAs help protect patients who are participating in clinical studies at more of an indirect level. This ideology helps prevent burnout, especially when monitors are swamped with the many reports that are necessary as part of the monitoring process.
Checklist for Transitioning from Research Coordinator to CRA
Table 4 has a checklist for determining whether one is ready to make the transition from research coordinator to CRA. Prior to applying for positions, the research coordinator must consider his/her stage in the behavior change model. Unless the research coordinator is ready to transition to a CRA position, he/she should not do it. Becoming a CRA can be difficult without two to five years of research experience in medical devices, pharma, or academia in some capacity. A research coordinator who wants to transition to a CRA should work closely with current CRAs who can provide mentoring and networking opportunities as well as exploring other networking avenues such as SOCRA and ACRP forums, LinkedIn, and also attending the annual events or local events put on by these organizations.
It is important for research coordinators to bolster their resumes by completing supplemental training or certifications. Resumes should be up-to-date and attractive to potential employers. This means including details about accomplishments along with basic information such as job titles and education.
The research coordinator must also consider the travel demands of a CRA position, the types of monitoring to pursue, and his/her stage in the behavior change model. Travel is a major part of a CRA position and should be a focal point of your conversation with a hiring representative. Finally, the types of monitoring including central monitoring, remote monitoring, and regional monitoring should be considered.
Monitoring is a great job. It allows a lot of freedom. However, CRAs also have a great deal of responsibility. CRAs must be driven, willing to put in the time, and have the necessary work ethic while maintaining vigilance and holding others accountable for good clinical practices.
Typical Background of a CRA
- Nursing degree with a clinical research background
- Bachelor’s or master’s degree in clinical research
- Former/current research coordinator
- Clinical experience (medical assistant, registered nurse, or nurse practitioner)
- Clinical research certified ( SOCRA CCRP ® or ACRP-CP)
- Research experience/background
- Science/academic research background
The Life of a CRA
- This requires being self-motivated and driven
- Sometimes performing a combination of onsite and remote monitoring
- Email, etc.
- At times, CRAs are gone for several days at a time depending on current workload
- Visit preparation and follow-up is a crucial part of work at home
Types of Monitoring
- Annual or semi-annual visits based upon the monitoring plan
- Risk-based monitoring/central monitoring
- Remote monitoring
- In-house CRAs and regulatory CRAs
- Sponsor CRA/monitor
- CRO CRA/monitor
Checklist for Transitioning from a Clinical Research Coordinator
to a Monitoring CRA (Clinical Research Associate)
- 2-5 years of research experience as a research coordinator or research assistant
- Able to work with current CRAs as part of a mentorship or network with CRAs
- Completion of supplemental training or certifications to support career goals and bolster resume
- Explore networking avenues
- Up-to-date resume that is attractive to potential employers
- Able to meet travel demands of a CRA position
- Consideration of types of monitoring to pursue
- Stage in the behavior change model
7 thoughts on “Career Progression in Clinical Research: Transitioning from a Clinical Research Coordinator to a Monitoring Clinical Research Associate (CRA)”
Your articles are always helpful and I always get something new to learn from them.
Research Update Organization
you are always giving something new. thank you for that.
Very clear and helpful article! The tables listing the different types of monitoring roles and the items to consider whether this is right transition are a great summary, too.
- Pingback: An overview of a career in clinical research: What jobs are available in this field? - Rebiz Zield
This is a very clear and collective article. The tables are the best helpful tips and resources for anyone interested for career advancement in clinical research. I really appreciate the author’s time and effort to sharing this article.
- Pingback: Career Growth Opportunities in Clinical Research
- Pingback: Introduction to Clinical Research: What Every Student Should Know - Drug Safety and Pharmacovigilance Course
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
2024 ANNUAL CONFERENCE
ADVANCING INNOVATION AND INTEGRITY:
A TIME FOR TRANSFORMATION IN CLINICAL RESEARCH

8 tracks I 20+ Topic Areas I 100+ Sessions I 70+CE

IMAGES
COMMENTS
Apr 5, 2024 · A comprehensive comparison of Clinical Research Assistants vs. Coordinators. Explore the difference between Clinical Research Assistants and Coordinators in their roles, responsibilities, skills, salary, and career growth opportunities.
Oct 4, 2023 · The Clinical Research Coordinator and Clinical Research Associate roles are different. However, because there can be some overlap, here are four key differences between a CRC and a CRA. CRCs and CRAs have different educational requirements.* A Clinical Research Coordinator can be an entry-level position.
Apr 18, 2021 · Difference between clinical research coordinator and clinical research associate: When a PI (principal investigator, i.e. often a busy, working physician running a trial on the side) is chosen to conduct a trial at their site, clinical research coordinators often take over part of the essential responsibilities of PI.
Wrapping Up: Comparing CRAs and CRCs in Clinical Research. In summary, the roles of the Clinical Research Associate (CRA) and the Clinical Research Coordinator (CRC) are distinct yet interconnected. CRAs focus on monitoring and ensuring compliance, while CRCs are responsible for participant coordination and data management.
Oct 5, 2022 · Here are the main differences between a research assistant and a research coordinator. Job Duties. Research assistants perform more hands-on tasks and research coordinators handle more administrative responsibilities. For example, a research assistant might interview study participants, administer questionnaires or conduct laboratory tests.
Jul 30, 2024 · Tips for choosing between clinical research coordinator or clinical research associate careers When you are interested in the clinical research field, you can compare the clinical research coordinator and associate professions to determine which option best suits you. You can use the following tips to guide your decision-making process:
Feb 13, 2023 · Research Assistant vs. Research Coordinator Job Duties. When it comes to educational and job experience, there are distinct differences between the roles of a research assistant and a research coordinator. Both positions can be found in academic, medical, and corporate research settings and require very specific skill sets.
Apr 5, 2024 · A clinical research coordinator is a healthcare professional responsible for administering clinical trials of drugs or medications. Clinical research coordinators work under the supervision of clinical research managers to collect data and help inform trial participants about the study's objectives.
Dec 14, 2021 · A research coordinator is a person at the clinical research site who is involved in the daily tasks of enrollment, data entry, and all other aspects of clinical trials at the site level. A clinical research associate (CRA), or monitor, is the individual who visits clinical research sites to review their medical records and do the standard ...
Feb 25, 2008 · I'm currently working as a Research Coordinator and I have worked as a Research Assistant in the past. The previous posts are right in that as a research coordinator you have more administrative responsibilities, such as recruiting, filing IRB forms, scheduling participants, but may also include research experience such as assisting with the study design, scoring of measures, data entry, data ...