The Good Genes Hypothesis
- Living reference work entry
- First Online: 13 January 2023
- Cite this living reference work entry

- Urszula M. Marcinkowska 2
508 Accesses
23 Altmetric
Honest signalling
The good genes hypothesis proposes that the characteristics preferred by females are a signal of males’ ability to pass on genes which will increase the survival and reproductive success of the offspring sired with a male possessing them.

Introduction
The good genes hypothesis (GGH) was formulated by evolutionary biologist W.D. Hamilton and behavioral ecologist M. Zuk ( 1982 ). It proposes that the characteristics preferred by females are a signal of males’ ability to pass on genes (coding that certain characteristic) which will increase the survival and reproductive success of the offspring sired with a male possessing them. In such case, sexual selection would lead to the increase of the proportion of genes (coding the attractive trait) that are beneficial under prevailing conditions. In other words, good genes increase fitness of the offspring. In an evolutionary and ecological understanding individual’s fitness depends on how many copies of own...
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Institutional subscriptions
Achorn, A. M., & Rosenthal, G. G. (2019). It’s not about him: Mismeasuring ‘good genes’ in sexual selection. Trends in Ecology & Evolution, 35 (3), 206–219. https://doi.org/10.1016/j.tree.2019.11.007
Boothroyd, L. G., Scott, I., Gray, A. W., Coombes, C. I., & Pound, N. (2013). Male facial masculinity as a Cue to health outcomes. Evolutionary Psychology, 11 (5), 1044–1058. https://doi.org/10.1177/147470491301100508
Article Google Scholar
Foo, Y. Z., Simmons, L. W., & Rhodes, G. (2017). Predictors of facial attractiveness and health in humans. Scientific Reports, 7 . https://doi.org/10.1038/srep39731
Hakkarainen, T. J., Krams, I., Coetzee, V., Skrinda, I., Kecko, S., Krama, T., … & Rantala, M. J. (2021). MHC class II heterozygosity associated with attractiveness of men and women. Evolutionary Psychology , 19 (1). https://doi.org/10.1177/1474704921991994 .
Hamilton, W. D., & Zuk, M. (1982). Heritable true fitness and bright birds: A role for parasites? Science, 218 (4570), 384–387. https://doi.org/10.1126/science.7123238
Marcinkowska, U. M., Ziomkiewicz, A., Kleisner, K., Galbarczyk, A., Klimek, M., Sancilio, A., … & Bribiescas, R. G. (2020). Oxidative stress as a hidden cost of attractiveness in postmenopausal women. Scientific Reports, 10 (1). https://doi.org/10.1038/s41598-020-76627-9 .
Prum, R. O. (2010). The Lande-Kirkpatrick mechanism is the null model of evolution by intersexual selection: Implications for meaning, honesty, and design in intersexual signals. Evolution, 64 (11), 3085–3100.
Rantala, M. J., Coetzee, V., Moore, F. R., Skrinda, I., Kecko, S., Krama, T., … & Krams, I. (2013). Adiposity, compared with masculinity, serves as a more valid Cue to immunocompetence in human mate choice. Proceedings of the Royal Society B-Biological Sciences, 280 (1751). https://doi.org/10.1098/rspb.2012.2495 .
Scott, I. M. L., Clark, A. P., Boothroyd, L. G., & Penton-Voak, I. S. (2013). Do Men’s faces really signal heritable immunocompetence? Behavioral Ecology, 24 (3), 579–589. https://doi.org/10.1093/beheco/ars092
Stephen, I. D., Hiew, V., Coetzee, V., Tiddeman, B. P., & Perrett, D. I. (2017). Facial shape analysis identifies valid Cues to aspects of physiological health in Caucasian, Asian, and African populations. Frontiers in Psychology, 8 . https://doi.org/10.3389/fpsyg.2017.01883
Weatherhead, P., & Robertson, R. (1979). Offspring quality and the polygyny threshold: “The sexy son hypothesis”. The American Naturalist, 113 (2), 201–208. https://doi.org/10.1086/283379
Zahavi, A. (1975). Mate selection—A selection for a handicap. Journal of Theoretical Biology, 53 (1), 205–214. https://doi.org/10.1016/0022-5193(75)90111-3
Zaidi, A. A., White, J. D., Mattern, B. C., Liebowitz, C. R., Puts, D. A., Claes, P., & Shriver, M. D. (2019). Facial masculinity does not appear to be a condition-dependent male ornament and does not reflect MHC heterozygosity in humans. Proceedings of the National Academy of Sciences of the United States of America, 116 (5), 1633–1638. https://doi.org/10.1073/pnas.1808659116
Download references
Author information
Authors and affiliations.
Institute of Public Health, Jagiellonian University Medical College, Cracow, Poland
Urszula M. Marcinkowska
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Department of Psychology, Oakland University, ROCHESTER, MN, USA
Todd K. Shackelford
Section Editor information
Department of Psychology, Oakland University, Rochester, MI, USA
Oakland University, Rochester, MI, USA
Gavin Vance
Department of Psychology, Oakland University, Rochester Hills, MI, USA
Madeleine K. Meehan
Rights and permissions
Reprints and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this entry
Cite this entry.
Marcinkowska, U.M. (2023). The Good Genes Hypothesis. In: Shackelford, T.K. (eds) Encyclopedia of Sexual Psychology and Behavior. Springer, Cham. https://doi.org/10.1007/978-3-031-08956-5_1081-1
Download citation
DOI : https://doi.org/10.1007/978-3-031-08956-5_1081-1
Received : 29 August 2022
Accepted : 29 August 2022
Published : 13 January 2023
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-08956-5
Online ISBN : 978-3-031-08956-5
eBook Packages : Living Reference Behavioral Science and Psychology Reference Module Humanities and Social Sciences Reference Module Business, Economics and Social Sciences
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Good genes hypothesis
The good genes hypothesis is a model of sexual selection that claims mate preferences for particular traits, e.g. for beauty , have evolved because these traits are reliable indicators of overall health and other 'good' genetic traits, [1] Thus, the theory claims, mate choice is not evolutionarily maladaptive or neutral, but beneficial and eugenic in terms population viability.
- 1 Good geners vs Fisherians
- 2 Incelosphere
- 3 Contemporary good geners
- 4 Homocel hypothesis
- 5 References
Good geners vs Fisherians [ edit | edit source ]
An alternative (but not necessarily contradicting [2] ) model of sexual selection and mate choice is Fisherian selection . This theory suggests the traits animals select for are mere ornament and that the ornament may even reduce population viability and thus mate choice adaptations may be maladaptive.
Research into these models of sexual selection has given rise to rivalry between Good Geners and Fisherians in academica. [3] [4] [5] Cronin (1991) called this debate the 'sexy sons' vs 'healthy offspring' debate. [6]
Incelosphere [ edit | edit source ]
Various forum users have different stances on the good genes hypothesis. Members of incels.co generally promote the theory, knowingly or unknowingly. Fschmidt 's incel forums define themselves as against the hypothesis (e.g. /r/nonmorons) and instead lean more toward strong Fisherian hypotheses and they think incels are instead 'genetically superior' because they think humanity is in a Fisherian runaway . People from Fschmidt and Caamibs circle generally dislike other forums for falsely giving the impression that incels are 'low-IQ'. Yourenotalone.co also leaned toward Fisherian hypotheses. Older forums such as IncelSupport generally considered dating effort the most consistent predictor of mating success instead of sexiness or 'good genes', a view wiki editor Altmark22 also holds.
Contemporary good geners [ edit | edit source ]
Many blackpillers promote good genes, whether knowingly or not. They see incels as subhumans or genetically inferior because of being rejected by women, from which some derive social Darwinist conclusions that incels should commit suicide as that's better for the gene pool.
Besides blackpillers, the good genes hypothesis is also promoted by e.g. Canadian professor Jordan Peterson as well as by modern (but not traditional) eugenicists and racialists such as Edward Dutton . However, Peterson is not Good Gener in all regards, e.g. he claimed women's inclination for mating with highly dominant men would be bad for society. [7]
Good geners go looking for reasons why female preferences might indicate 'good genes', for example by saying women prefer symmetrical faces because symmetry is an honest signal of developmental stability, low mutational load and hence overall genetic fitness . Further, developmental stability is thought to be necessary for overall health. Taken together, one would expect that symmetry and health are correlated in the overall population. However, several studies did not find such a link:
Contrary to the hypothesis that symmetry cues health, the largest study of facial asymmetry and health to date found no relationship between these variables. Researchers used data from a British cohort study of 4732 individuals and found that facial symmetry at age 15 was unrelated to longitudinal measures of childhood health, including measures of the proportion of childhood years spent unwell, average number of illness symptoms per year, and total number of infections. [8]
Even though facial attractiveness is correlated with health (see beauty ), and even though bad genes may be visible in extreme cases such as disfigurement, certain genetic conditions such as down syndrome, these kinds of links appear to be very weak overall.
Good geners tend to contradict themselves though by also implying that incels are overlooked irrationally by women, unless they are only speaking of specific incels or are ok with what they would think would be dysgenics if incels were not overlooked.
Some, but not all, feminists also promote the good genes hypothesis by insinuating that sexual selection outside of patriarchy is a moral and rational sorter of good and bad genes, including a feminist who held a prominent position in the FBI. [9] [10] Feminists more often take issue with Darwin in general though, [11] so these overtures are infrequent and often passively expressed in arguments, if at all.
A minority of journalists, such as Brian Clarey signal explicit belief in the good genes hypothesis.
Homocel hypothesis [ edit | edit source ]
The prevalence of outright Chad worship among some blackpilled incels appears to have a homoerotic undertone , reminding of the homocel hypothesis . Chad worship may ultimately be driven by a desire to get sodomized by Chad after telling him how great his genes are in order to get some crumbs in return.
References [ edit | edit source ]
- ↑ https://onlinelibrary.wiley.com/doi/full/10.1111/j.1558-5646.2012.01654.x
- ↑ https://www.researchgate.net/publication/327130679_The_Evolution_of_Feminine_Beauty
- ↑ https://books.google.com/books?id=9Ni9ggiew1UC&pg=PA65&lpg=PA65&dq=%22good+geners%22&source=bl&ots=R85Bx3GjIu&sig=ACfU3U38KkFqtKlY1XHb-EhWugFBWk0GgQ&hl=en&sa=X&ved=2ahUKEwj1qMqRqY7pAhXIgXIEHZOfAVcQ6AEwAHoECAgQAQ#v=onepage&q&f=false
- ↑ https://oneclass.com/textbook-notes/ca/western/psyc/psy-3229ab/197622-chapter-3pdf.en.html
- ↑ http://flax.nzdl.org/greenstone3/flax?c=BAWESS&a=d&rt=r&d=D2155&dt=simple&p.a=b&p.s=ClassifierBrowse&cls=
- ↑ https://www.youtube.com/watch?v=OeL-Fn0V8iU
- ↑ https://royalsocietypublishing.org/doi/pdf/10.1098/rstb.2015.0380
- ↑ https://twitter.com/DCELL68/status/992147364084310016 [ Archive.is ]
- ↑ https://twitter.com/CrazyEddyBee/status/1227808113878872065 [ Archive.fo ]
- ↑ https://www.smithsonianmag.com/science-nature/woman-who-tried-take-down-darwin-180967146/
See also [ edit | edit source ]
- Fisherian runaway
- Genetic dead end
Navigation menu

GOOD GENES HYPOTHESIS
Hypothesis of female mate selection that argues genetic variation in males correlates with success, features of male behaviour provide information about variation , females respond to variation by choosing males with good genes.

Leave a Reply
Your email address will not be published. Required fields are marked *
Latest Posts

What Happens At An ADHD Assessment

A Quick Look at the History Behind Hypnosis

A Brief History of Brainwashing: The Science of Thought Control

A Deep Dive into the Social Psychology of Leadership

Counseling Approaches to Client Care: Theories to Apply in Practice

The Future Of Education: Can You Earn A Psychology Degree Online?

Insomnia & Mental Illness: What is the Correlation?

Stop Guessing: Here Are 3 Steps to Data-Driven Psychological Decisions


Getting Help with Grief: Understanding Therapy & How It Can Help

Exploring the Psychology of Risk and Reward

Understanding ADHD in Women: Symptoms, Treatment & Support

Meeting the Milestones: A Guide to Piaget's Child Developmental Stages
Popular psychology terms, medical model, hypermnesia, affirmation, backup reinforcer, brainwashing, affiliative behavior, message-learning approach, social instinct, personal adjustment, excitation-transfer theory, posttraumatic stress disorder (ptsd).
study guides for every class
That actually explain what's on your next test, good genes hypothesis, from class:, general biology i.
The good genes hypothesis suggests that certain traits are favored in mate selection because they indicate superior genetic quality. These traits increase the likelihood of offspring survival and reproduction.
congrats on reading the definition of good genes hypothesis . now let's actually learn it.
5 Must Know Facts For Your Next Test
- The hypothesis is often studied in the context of sexual selection and mate choice.
- Traits indicating 'good genes' can include physical characteristics, behaviors, or other phenotypic expressions.
- Good genes can confer advantages such as disease resistance, better metabolism, or more efficient resource use.
- Females may prefer males with these traits because their offspring are likely to inherit these advantageous qualities.
- Empirical evidence for the good genes hypothesis has been observed in various species, including birds, fish, and mammals.
Review Questions
- What does the good genes hypothesis propose about mate selection?
- How do 'good genes' benefit offspring according to this hypothesis?
- Can you name a type of trait that might be considered an indicator of good genes?
Related terms
Sexual Selection : The process by which certain traits increase an individual's chances of mating and reproducing based on attractiveness to potential mates
Mate Choice : The criteria individuals use to select their partners for reproduction
Phenotype : The observable characteristics or traits of an organism resulting from the interaction of its genotype with the environment
" Good genes hypothesis " also found in:
Subjects ( 1 ).
- Animal Behavior
© 2024 Fiveable Inc. All rights reserved.
Ap® and sat® are trademarks registered by the college board, which is not affiliated with, and does not endorse this website..
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Should females prefer old males?
Julia carolina segami, martin i lind, anna qvarnström.
- Author information
- Article notes
- Copyright and License information
E‐mail: [email protected]
Shared first authorship.
Corresponding author.
Revised 2021 Jul 1; Received 2020 Sep 3; Accepted 2021 Jul 12; Collection date 2021 Oct.
This is an open access article under the terms of the http://creativecommons.org/licenses/by/4.0/ License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Whether females should prefer to mate with old males is controversial. Old males may sire offspring of low quality because of an aging germline, but their proven ability to reach an old age can also be an excellent indicator of superior genetic quality, especially in natural populations. These genetic effects are, however, hard to study in nature, because they are often confounded with direct benefits offered by old males to the female, such as experience and high territory quality. We, therefore, used naturally occurring extra‐pair young to disentangle different aspects of male age on female fitness in a natural population of collared flycatchers because any difference between within‐ and extra‐pair young within a nest should be caused by paternal genetic effects only. Based on 18 years of long‐term data, we found that females paired with older males as social partners experienced an overall reproductive advantage. However, offspring sired by old males were of lower quality as compared to their extra‐pair half‐siblings, whereas the opposite was found in nests attended by young males. These results imply a negative genetic effect of old paternal age, given that extra‐pair males are competitive middle‐age males. Thus, offspring may benefit from being sired by young males but raised by old males, to maximize both genetic and direct effects. Our results show that direct and genetic benefits from pairing with old males may act in opposing directions and that the quality of the germline may deteriorate before other signs of senescence become obvious.
Keywords: Ageing, direct benefits, extra‐pair mating, female preference, genetic benefits, germline senescence, mate choice, reproductive senescence
Impact summary
Male age may influence the number and quality of the offspring he produces. This is due to senescence, the gradual deterioration of organism function with age, which can affect the germline cells quality (i.e., genetic or indirect parental effects) and the ability to provide resources (i.e., direct parental effects). However, proven ability to survive until old age may mean that older fathers instead will produce high‐genetic‐quality offspring, able to survive, and get old themselves. Additionally, old males may also be able to provide their offspring with better or more resources due to gained experience. These reproductive pros and cons of old male partners impose a dilemma for mate choosing females. Should they avoid or prefer old males to ensure direct and indirect benefits? This question has not yet been fully answered because direct and indirect effects of paternal age are hard to separate in the wild. We circumvented this problem by comparing nestlings sired by different males of different ages within the same brood. Therefore, we were able to distinguish the indirect (genetic) from direct (environmental) effects of biological fathers, as the environment and maternal genes were constant. We used 18 years of long‐term data on a collared flycatcher population of which 812 individuals were genotyped to assign paternity. We found that direct benefits increase with male age, but at the same time the germline quality decreases. Moreover, this deterioration happens before other signs of senescence become evident. These findings have profound consequences for the general understanding of the evolution of mate choice, which is rarely considered in the context of senescence. Our study is one of very few exploring the effects of male senescence in the wild and it is the first one to disentangle genetic from direct benefits in the context of mate choice.
Ageing is the physiological deterioration of organism functionwith age and is widespreadin nature (Shefferson et al. 2017 ). Increasing age can affect fitness of the individual (Lemaître and Gaillard 2017 ) and also its offspring through negative parental age affects (Monaghan et al. 2020 ), either because of reduced general phenotypic performance or because of accumulation of deleterious germline mutations. Therefore, age could influence partner choice and females may avoid mating with old males to minimize these negative effects. However, there are also numerous positive effects associated with increased age. In fact, females are often assumed to prefer to mate with older males to gain access to an experienced mate with superior resources and proved ability to survive (Grant and Grant 1987 ; Conner 1989 ; Côté and Hunte 1993 ; Takagi 2003 ; Dupont et al. 2018 ). How these negative and positive aspects of male age translate into number and quality of the offspring they sire and/or raise and hence influence the fitness of females that have chosen to breed with them remain open questions. Whether females should prefer to mate and pair with old males has therefore become a controversial subject (Brooks and Kemp 2001 ; Griffith et al. 2002 ; Beck and Promislow 2007 ; Dean et al. 2010 ; Lifjeld et al. 2011 ; Dupont et al. 2018 ; Rodríguez‐Muñoz et al. 2019 ). Accordingly, there is a need to investigate whether detrimental effects of old age are common in males and also to examine to what extent such effects are strong enough to compromise the reproductive success of females in the wild (Lemaître and Gaillard 2017 ).
Traditionally, natural selection was thought to favor females that prefer older males because such males should be more experienced and thereby also more likely to have access to high‐quality resources and to provide better parental care, which would provide direct benefits to the female (Grant and Grant 1987 ; Conner 1989 ; Côté and Hunte 1993 ; Takagi 2003 ; Dupont et al. 2018 ). Moreover, mating with older males can also give indirect (genetic) benefits to the female in terms of more fit offspring. The “good genes” hypothesis predicts a preference for older individuals based on the logic that survival until older ages requires high‐quality genes, which in turn will be passed on to the offspring (Trivers 1972 ; Kokko and Lindstrom 1996 ; Kokko 1998 ; Bouwman et al. 2007 ). In accordance with these expectations, there are several studies demonstrating that older males are more likely to attract females for mating (Conner 1989 ; Côté and Hunte 1993 ), to become socially paired (Weatherhead 1984 ; Alatalo et al. 1986 ), to be less likely to lose paternity in the broods they attend (Michálková et al. 2019 ), and to be more likely to gain extra pair paternity (Dickinson 2001 ; Bouwman et al. 2007 ; Cleasby and Nakagawa 2012 ; Michálková et al. 2019 ).
Possible negative aspects of pairing with relatively old males can be both in terms of reduced phenotypic skills (i.e., reduced resource holding potential or ability to provide parental care) as a consequence of senescence of the soma, but also genetically in terms of mutation accumulation in the male germline. Physiological senescence with increasing age is widespread across organisms (Shefferson et al. 2017 ), and examples from birds that could result in direct costs are reduced to foraging efficiency and nest defense (Newton and Rothery 2002 ; Bouwhuis and Vedder 2017 ). In contrast, purely genetic costs to the offspring of old fathers stayed for a long time undetected in wild animals. This contrasts to laboratory studies, where offspring from old male mice were found to have decreased reproduction and longevity (García‐Palomares et al. 2009 ), and negative paternal age effects on offspring life span have also been found in Drosophila (Priest et al. 2002 ) and captive populations of zebra finch (Noguera et al. 2018 ). Moreover, in humans, offspring life span decreases with increasing age of the father (Gavrilov and Gavrilova 1997 ; Kemkes‐Grottenthaler 2004 ). Such negative effects of male age were for a long time considered irrelevant in the context of evolution of mate choice in nature. Predation and other sources of mortality were thought to remove individuals from the population before the onset of senescence (Medawar 1951 ; Comfort 2011 ). Moreover, even if a few males would survive until the onset of senescence the likelihood of mating with them would be minimal meaning that selection on females to avoid mating with too old males should be negligible (Finch 1998 ). The few males that do show senescence may also compensate by being more experienced at acquiring resources or providing parental care further lowering the potential gain of avoiding these males as mates. However, these arguments have recently become questioned.
Because the reproductive success of males is very much determined by the ability to secure a mate and sire as many offspring as possible, males are expected to invest in secondary sexual characters, expensive behaviors (such as male‐male interactions, courtship, territory defense), and/or sperm competition, perhaps even at the expense of their germline maintenance (Lemaître and Gaillard 2017 ). Germline cells deteriorate with advanced age (Kong et al. 2012 ) and mutation accumulation in the germline cells can have negative effects on the quality of the offspring in natural populations (Pizzari et al. 2008 ; Velando et al. 2011 ). Mutation rates have also been shown to be higher in males than in females (Kong et al. 2012 ; Smeds et al. 2016 ) probably due to the large number of cell divisions during spermatogenesis. Moreover, recent findings in Drosophila suggest that the fitness effects of negative mutations often increase nonlinearly with age (Brengdahl et al. 2020 ). Thus, it is possible that effects of male senescence on offspring number and quality, especially in the form of mutation accumulation in the male germline, have been overlooked in natural populations. There is now growing evidence that various effects of senescence may be observed also in natural populations (Bonduriansky and Brassil 2002 ; Nussey et al. 2013 ; Bouwhuis and Vedder 2017 ; Froy et al. 2019 ; Gaillard and Lemaître 2020 ), which suggests that negative effects of advanced age can be important for mating decisions, at least if senescent males are present in the population or if the germline deteriorates before other visible signs of ageing occur. Still, relatively few studies have investigated possible effects of male reproductive senescence in the wild (Lemaître and Gaillard 2017 ), with a study on house sparrows being a rare exception. This study focused solely on possible genetic effects by using a cross‐fostering design and found that offspring of old parents had lower reproductive success, but with a sex‐specific effect such that old paternal age only influenced the fitness of sons and old maternal age only affected the fitness of daughters (Schroeder et al. 2015 ).
Taken together, the decision to mate with an old male reflects a balance between positive direct (Grant and Grant 1987 ; Conner 1989 ; Côté and Hunte 1993 ; Takagi 2003 ; Dupont et al. 2018 ) and genetic (Trivers 1972 ; Kokko and Lindstrom 1996 ; Bouwman et al. 2007 ) benefits of choosing an old mate, but also potential costs associated with mating with too old males that experience senescence of the soma (Bouwhuis and Vedder 2017 ) or germline (Pizzari et al. 2008 ; Velando et al. 2011 ; Kong et al. 2012 ), which are outlined in Figure 1 . How these different aspects of male age translate into number and quality of the offspring they sire and/or raise and hence influence the fitness of females that have chosen to breed with them remain open questions. The relative importance of these different effects has to our knowledge not previously been disentangled in studies on natural populations. Our study aims to fill this knowledge gap using two approaches: (1) using long‐term breeding data of collared flycatchers ( Ficedula albicollis ) to assess the effect of paternal age on offspring fledge number and offspring recruitment and (2) by using naturally occurring extra‐pair offspring to disentangle direct and genetic effects of paternal age on offspring quality while keeping maternal genetic effects constant. Collared flycatchers are small passerine birds that preferably breed in nest boxes which allows long term monitoring. The flycatcher population of Öland has been monitored over 18 years and pedigrees as well as age records are available since 2002 (Qvarnström et al. 2010 ). We used these long‐term breeding data to test whether females benefit from breeding with older males. In addition, we also genotyped a large number of offspring to specifically single out possible genetic effects associated with paternal age such as increased genetic quality of offspring with male age due to the male's proven ability to survive and/or decreased genetic quality of offspring with male age due to germline deterioration. In this case, we only have information of the age of the social male as the extra‐pair male is unknown. Nevertheless, we can infer the relative age of the extra‐pair male based on two facts. First, most extra‐pair young (EPY) are known to be sired by competitive middle‐aged males as revelated by other studies (Dickinson 2001 ; Bouwman et al. 2007 ; Cleasby and Nakagawa 2012 ; Michálková et al. 2019 ). Second, even if females would randomly mate with extra‐pair males the age structure of the studied population indicates that young males breeding for their first time would be cuckolded by equally old or older males (i.e., on average older males). Similarly, males belonging to the oldest age classes should be cuckolded by younger males.
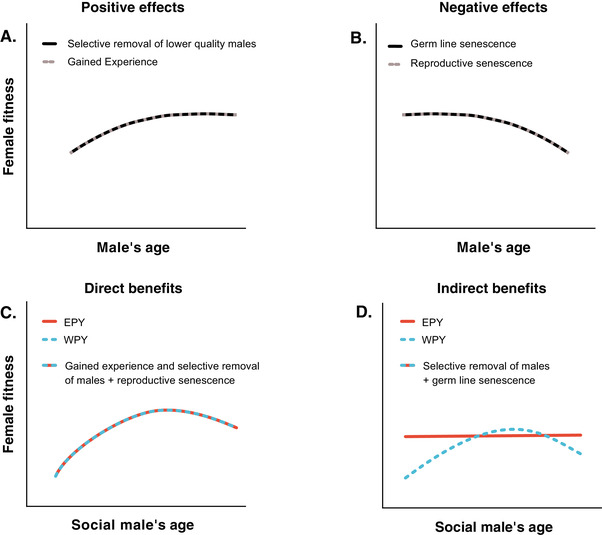
The first two panels show expected relationships between female fitness in terms of number and quality of offspring produced and the age of her mate through several possible processes. The second two panels illustrate how naturally occurring extra pair young can be used to disentangle effects mediated by resources or genes provided by the males. This is because these offspring share the same nest environment, including resources provided by the social male, and maternal genes but are sired by males of (on average) different ages. (A) Positive effects of male age on female fitness. Increased male experience and/or selective removal of males of low quality may lead to higher quality of the resources such as territories and paternal care that are provided by the older males. Selective removal of low‐quality males may also ensure genetic benefits to females selecting older males that have proven their ability to survive. The expected net benefits to females with increasing male age will level off as selection will have removed the males of lowest quality and due to an expected less sharp learning curve late in life. (B) Negative effects of male age on female fitness. Reproductive senescence may negatively affect the phenotypic quality of males toward the end or their lives, leading to middle‐aged males being of highest phenotypic quality and therefore providing the best direct benefits to their females. Old males may also experience decreased germline quality as deleterious mutations accumulate with male age leading to a decline in genetic benefits obtained by females selecting older males as mates. These negative fitness effects are expected to accelerate in association with “tipping points” when a decline in male general phenotypic quality, for example, makes them unable to defend high‐quality territories. (C) Direct material benefits from socially pairing with males of different ages will have similar effects on the number and phenotypic quality of within‐ (WPY) and extra‐pair young (EPY). (D) Assuming that extra‐pair young are mainly sired by middle‐aged competitive males, we expect EPY to be of superior genetic quality as compared to WPY when the social male is very young or very old. This is because very young social males have not yet proven their ability to survive to older ages (i.e., selection has not yet removed low‐quality males) and very old social males may experience germline senescence.
DATA COLLECTION
The collared flycatcher ( Ficedula albicollis ) is a small migratory passerine of the family Muscicapidae ; they overwinter in sub‐Saharan Africa and breed in Europe (Qvarnström, Rice, and Ellegren 2010 ). At the beginning of the reproductive season in early May, males arrive to the breeding grounds and establish territories that they advertise to females. Once a female starts building the nest, the male defends its territory from other males and predators. After laying eggs, the female starts incubating and the male provides her food. When the eggs hatch, both the male and the female share the task of feeding the nestlings and guarding the nest. They are insectivorous with a preference for caterpillar larvae as food for their offspring (Shirihai and Svensson 2018 ). The population breeding on Öland (57°100N, 16°580E) has been monitored in deciduous and mixed forests with over 2000 nest boxes across the island during the reproductive season between May and July since 2002 (Qvarnström, Rice, and Ellegren 2010 ). Metal rings with a unique identity code are placed on every bird and morphological measurements as well as a blood samples are taken every year. Additionally, the offspring are weighted (±0.1g) at day 6 and day 12 before fledgling with a spring Pesola of 30 g. This long‐term data collection allowed us to determine social pedigrees, age, and fitness measurements. In addition, we used a subset of breeding events to specifically test for possible effects of paternal age on the genetic quality of the offspring. We genotyped 812 offspring from 154 nests. These nests are from the following years: 2002, 2004–2005, 2008, and 2010–2016.
To assess extra‐pair and within‐pair paternity of social couples offspring, we compared 12 microsatellite loci (FhU1, FhU2, FhU3, FhU4, Fhy223, Fhy301, Fhy304, Fh401, Fhy403, Fhy407, Fhy454, and PdoU5) of the offspring with their known mother and their social father using the software Cervus 3.0.3 (Marshall et al. 1998 ; Kalinowski et al. 2007 ) as previously described in similar studies (Alund et al. 2013 , 2018 ). For this analysis, data were simulated for 10,000 offspring with five candidate fathers assuming the sampling of 70% of the population (Jones et al. 2010 ). Only individuals where at least six microsatellite loci were compared were included and the confidence level used to establish extra pair paternity in the pairwise comparison between offspring and social father was >95%. It was not possible to identify the extra‐pair sires.
STATISTICAL ANALYSIS
Our overall strategy for statistical analyses was to construct a limited number of biologically relevant models to test the hypotheses outlined in Figure 1 . These models are presented in Table S1 . In general, these models include both a linear and quadratic effect of male age, because nonlinear fitness effects of age may be present for both direct (Forslund and Pärt 1995 ) and genetic effects (Fig. 1D ) (Pizzari et al. 2008 ). These linear and nonlinear fitness effects are also expected to interact with predictors of interests. For offspring recruitment probability, we expect that the effect of male survival to next year can interact with age and age 2 due to expected age‐dependent solutions to life‐history trade‐offs (Clutton‐Brock 1984 ). We also expect that the effect of EPY status on offspring mass can differ depending upon parental age, in both a linear and nonlinear fashion. Moreover, although reproductive data are often underdispersed and arguments have been raised to analyze this type of data using flexible generalized linear models (Brooks et al. 2019 ), other arguments favor the robustness and interpretability of Gaussian models for this type of data (Knief and Forstmeier 2020 ). We therefore analyzed reproductive data using both methods, and because our results are robust to the method used, we present the Gaussian models in the Results section and the corresponding generalized linear models in the Supporting Information.
Long‐term breeding dataset
To understand the age structure of the population, data of individuals of known age (i.e., ringed the year of birth or as 1‐year old when plumage reveals exact age of the male) between 2002 and 2018 were visually explored with a population pyramid plot and age percentages of different age classes were calculated.
We analyzed the effect of the social fathers age on the number of fledged offspring (i.e., offspring who left the nest alive) and number of recruits (i.e., offspring of the given clutch that returned after migration to breed in the population on subsequent years), using both cross‐sectional data and longitudinal data of 1‐year‐old individuals to test for selection between age class 1 and 2. These males ( n = 1094) were monitored between 2002 and 2018, and the total number of broods is 1527 of which 117 had 0 fledglings. All experimental (manipulated) broods in the study area were excluded for these analyses. We constructed separate linear mixed‐effect models with fledgling number or number of recruits as response variables. We fitted the age of the social father as explanatory variable and also age 2 , to investigate any nonlinear effect of age. For that purpose, age was mean centered before calculating age 2 . Male ID and year were included in the models as random effects on the intercept. Because both number of fledgling and number of recruits are count data that are generally underdispersed, we used both Gaussian distribution of the package lme4 (Bates et al. 2015 ) and the “genpois” family distribution available in the glmmTMB package (Brooks et al. 2017 ). Because we got consistent results with both distributions, the Gaussian distribution models are presented in the Results section and the correspondent models fitted with glmmTMB can be found in the Supporting Information.
To test whether recruitment does not only depend on age but also on the survival of the parent to the next age category, we constructed a generalized mixed‐effect model with binomial distribution. Offspring recruitment (fail, success) was fit as response variable and age, age 2 , and male survival to the next year as explanatory variables, including all interactions. Male ID and year were included as random effects.
To determine whether mass at time of fledge is a good predictor for recruitment in our population, we constructed a generalized linear mixed‐effect model with binomial distribution having successful or unsuccessful recruitment as a response variable. Mass at day 12 (briefly before fledgling) and lay date were fitted as explanatory variables, whereas nest ID and year were fitted as random effects.
Extra‐pair offspring dataset
To determine the effect of the age of the social father on the offspring's weight at fledgling, we constructed a linear mixed‐effect model with the weight of the offspring at day 12 as the response variable. As explanatory variables we included the paternity status (EPY or within‐pair young [WPY]) and the number of nestlings in the nest, because there is evidence that this is an important factor influencing nestling weight (Källander and Smith 1990 ). We also fitted father's age and age 2 . Year and male ID were included as random effects on the intercept, male ID was then removed as it did not explain any variation in the model and caused singularity problems to the Gaussian model (male ID was retained in the Generalized model [Table S14 ], where it did not cause singularity issues). To avoid pseudo replication because individuals from the same brood can share the same father, we included a random slope of brood ID over paternity status. All statistical analyses were conducted in R 3.5.3 (R Core Team 2019 ), mixed‐effect Gaussian and binomial models were implemented using the package lme4 (Bates et al. 2015 ), and Poisson models were implemented using the package glmmTMB (Brooks et al. 2017 ). All plots were created with the package ggplot2 (Wickham 2016 ) and show the last age category grouped when and additional category would yield less than 5% of all the data points. Significance was assessed using confidence intervals that were obtained using the confint function with Wald method.
The population shows a pyramidal age structure, where females and males show a similar age distribution with the majority of the population being composed by birds of 1 (45.8%) and 2 (25.5%) years old (Fig. S1 ; Table S2 ). Every age category experiences an approximate 50% reduction and thus, we find very few individuals of age 4 (8.5%) or older (5.3%). These older individuals are approximately 6.5% of the male population and 4% of the female population with the oldest individual registered of 9 years old for males and 8 years old for females.
PATERNAL AGE AND OVERALL REPRODUCTIVE PERFORMANCE
Direct effects of the age of the social partner influence the number and quality of offspring that a female produces in the same way regardless of whether these offspring are WPY or EPY. Based on gained experience and selective removal of males with poor phenotypic quality (Fig. 1A ), we expected females to experience higher reproductive performance when breeding together with older males. We found that females paired with older males as their social partners produced more (0.25 ± 0.06, t = 4.51, P < 0.001; Table S3 ; Fig. 2A ) fledged offspring and more recruits that returned to the breeding population as adults themselves (0.06 ± 0.02, t = 3.53, P < 0.001; Table S5 ; Fig. 2B ). In addition to the linear effect of the social fathers age on the number of fledglings, a significant quadratic effect of age captures the curvature of the response, where the positive effect of increased age of the social father weakens after age 2 (Table S3 ; Fig. 2 ). The increased benefit from breeding with older males was hence mainly driven by a lower performance of females paired with 1‐year‐old males that were breeding for the first time (Fig. 2 ). This increased performance observed between the first and second year of breeding in the cross‐sectional data can be driven either by increased performance of males due to gained experience or by selective removal of males of relatively poor quality. To disentangle these two possibilities, we therefore re‐analyzed the dataset using only 1‐year‐old individuals that were known to survive until their second year of breeding or after. Both fledglings and recruitment models lose the significant effect of age 2 , the linear effect of age is only borderline significant (depending upon model), and importantly the parameter estimate of age is much closer to zero in the longitudinal dataset ( Tables S7 and S9 ). These results imply that selective removal of low‐quality individuals between the first and second breeding attempts plays an important role for explaining the benefits associated with selecting older males as breeding partners (Fig. 2 ).
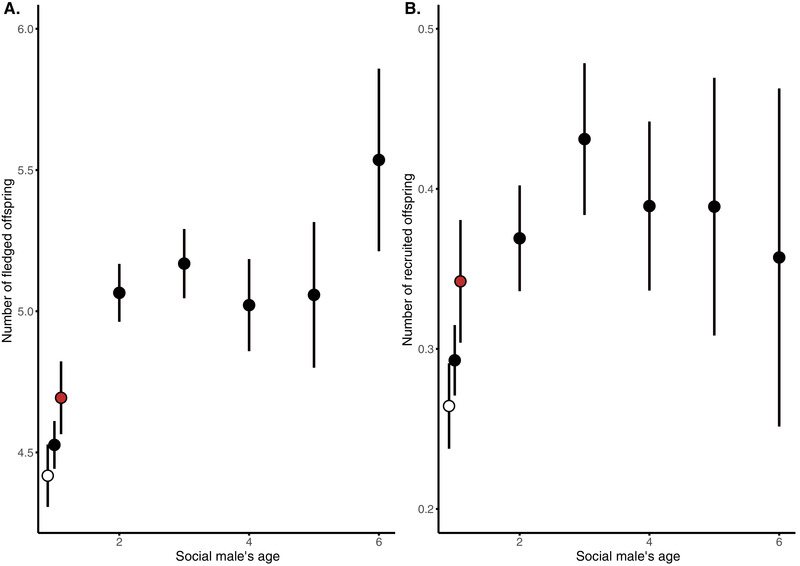
The number of fledglings (A) and recruits back into the breeding population (B) as a function of the social male's age in collared flycatchers. Symbols represent mean ± SE of the number of fledged offspring depending on the social male's age. Red symbol indicates data on males of age 1 that also survived to year 2, therefore controlling for selective disappearance. The white symbol indicates data on the males of age 1 who did not survive to year 4. There is a significant positive relationship between male age and reproductive performance that levels off with increasing age, but there is no apparent evidence for negative effects of male senescence on reproductive output when genetic and material effects are entangled.
In addition, we also analyzed the probability that a fledged offspring would recruit into the breeding population. For our a priori model, which included a linear as well as quadratic effect of age and all interactions, we found no relationship between male survival to next year and offspring recruitment probability after fledgling (Table S13 ). However, if we allow for model simplification using AIC, the best model (which excludes interactions) showed that offspring in the nest of older males (−0.13 ± 0.05, Z = –2.49, P = 0.013; Table S12 ; Fig. 3 ) and males who survive to the next year (−0.21 ± 0.1, Z = −2.18, P = 0.03; Table S12 ; Fig. 3 ) have a higher recruitment probability.
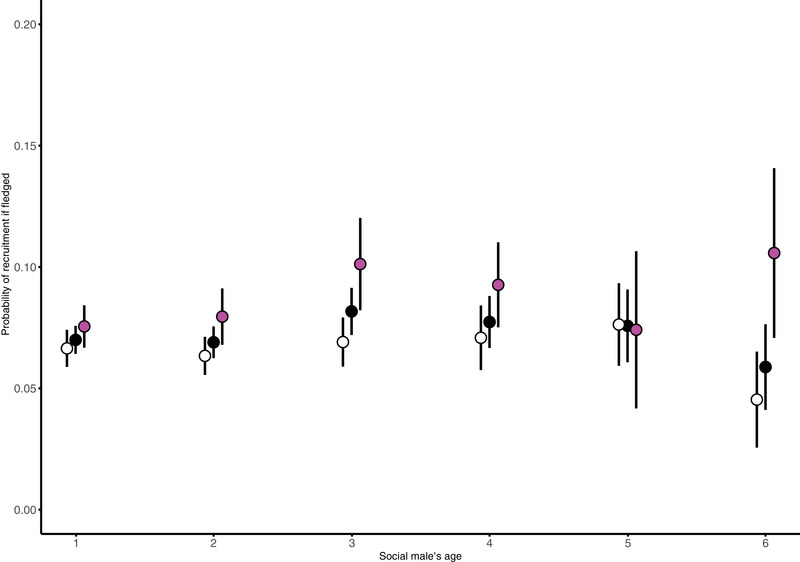
Probability of recruitment given successful fledgling depending on social male's age and survival to the next year. White symbols represent the mean probability ± SE of recruitment of offspring raised by males that died, magenta dots represent the mean probability ± SE of recruitment of offspring raised by males that survived, and black dots represent the mean ± SE of both. Offspring are more likely to recruit to the breeding population when they have fledged from nests attended by older males and males that survived to the following year themselves.
PATERNAL AGE AND GENETIC CONTRIBUTION TO OFFSPRING CONDITION
To estimate offspring quality, we used mass at day 12, which significantly predicts recruitment back to the breeding population (0.268 ± 0.026, Z = 10.116, P < 0.001, Table S11 ). We used 812 offspring from 154 social nests for which both the social male's age and paternity in the brood were known to disentangle possible effects of paternal age on the genetic quality of the offspring from direct effects of paternal age. We found a significant interaction between EPY status and age 2 (Table 1 ) on offspring mass (condition). The interaction between EPY status and age should be interpreted as a more or less negligible linear effect on age for both EPY (nonsignificant weakly negative) and WPY (estimated to be flat), whereas the interaction between EPY status and age 2 highlights fundamental differences in the nonlinear response, where EPY has a strong convex relationship between age and offspring mass with a minimum in young age and increased mass both for age 1 and especially age 4. In contrast, the quadratic relationship is convex and closer to zero for WPY. These interactions are illustrated in Figure 4 , showing that WPY have a relatively higher mass than EPY in nests attended by 1‐ and especially 2‐year‐old males. In nests attended by very old males (4 year or older), we find that WPY have a relatively lower mass than EPY (Table 1 ; Fig. 4 ). Because competitive middle‐aged males mainly sire the EPY, both these results imply a negative genetic effect of paternal age on offspring mass as predicted based on age‐dependent germline deterioration (Fig. 1D ).
Linear mixed‐effect model with offspring mass at day 12 as a responsevariable
The explanatory variables are Age of the social father (Age), Paternity status (either within‐pair offspring [WPY] or extra‐pair offspring [EPY]), and total number of offspring in the nest. As random effects, we have Year and a random slope for Paternity status on Nest ID. Number of observations: 812. Number of broods: 154. A confidence interval that does not overlap with 0 indicates significance for the value in this case the Estimate. And in the P column all values of p <0.05 are considered significant and hence they are in bold. P <0.05 in all cases matches the significance given by the calculation of confidence intervals.
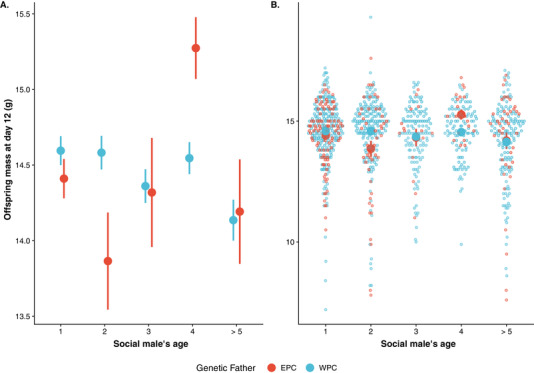
Mass at day 12 of extra‐pair (EPY, red color) and within‐pair (WPY, blue color) offspring depending on the social male's age. Within‐pair offspring are relatively heavier when the social male is young, but the opposite pattern with relatively heavier extra‐pair young is found when the social male is old. Offspring of males of age 5 or older are grouped in the plot but not on the analysis. (A) Mean ± SE of each age group. (B) Visualization of raw data points (with mean ± SE superimposed on top).
We found that female collared flycatchers benefit from breeding with old males, and especially should avoid 1‐year‐old males as social partners, to ensure overall high reproductive output. There was no evidence of reproductive male senescence lowering the direct, material benefits for females pairing with very old males. However, a comparison between WPY and EPY within a clutch revealed evidence consistent with deterioration of the germline of old males. Extra‐pair offspring had higher weight than within‐pair offspring in nest attended by old social males. Because old males are rare in the population, we assume that the EPY in these nests were sired by on average younger males. This result therefore demonstrates a decoupling of direct (material) and indirect (genetic) benefits of pairing with old males. Thus, female collared flycatchers could maximize their fitness by having their offspring sired by young males but raised by old males, where the direct benefits have an overall stronger effect on female fitness. Below we discuss these findings in more detail together with possible constraints on optimal female mating strategies.
The decision to mate with a male of a particular age reflects a balance of both direct and indirect benefits and costs associated with male age. Direct (or material) benefits are associated with resource provisioning and nest defense, and older males can often provide more resources due to increased experience and access to higher quality territories (Grant and Grant 1987 ; Conner 1989 ; Forslund and Pärt 1995 ; Takagi 2003 ). Indirect effects are instead related to the genetic effects of choosing a particular male, in terms of increased genetic quality of the offspring (Kempenaers and Dhondt 1993 ; Jennions and Petrie 2000 ; Griffith et al. 2002 ). Direct and genetic benefits are hard to disentangle in the wild, because both effects contribute to reproductive success of the social male, but we achieved this goal by using naturally occurring extra‐pair offspring. Any difference in performance between half‐sib WPY and EPY in a nest should be caused by genetic effects only, because males are not able to recognize its genetic offspring and therefore do not provide differential paternal care (Kempenaers and Sheldon 1996 ).
Firstly, we investigated the direct effect of paternal age. We found that females should prefer older males as social partners, and specifically avoid choosing young males as social partners. Both the number of fledglings and recruits (offspring returning to the breeding populations) increased with male age, which agree with a number of studies finding improved reproductive performance with age in birds (reviewed in Forslund and Pärt 1995 ). A significant nonlinear decline in the positive effect of male age suggested that the result was, as expected, mainly driven by low performance of 1‐year‐old males. Several mechanisms can underlie such patterns, such as increased experience (a direct effect), but also selective disappearance of low‐condition individuals, as well as age‐assortative mating (Zhang et al. 2015 ; Bouwhuis and Vedder 2017 ). By analyzing a longitudinal dataset with only individuals that survived at least 2 years, we found the effect of paternal age on the number of hatchlings and recruits almost disappeared, therefore selective disappearance of year‐1 males rather than increased experience is causing the increased reproductive performance of females having older males as their social partners. This result also excludes age‐assortative mating (where males and females of the same age preferentially breed together) as a possible explanation, because males surviving more than 1 year had a relatively high reproductive performance already as young. Moreover, we also found that the probability that a fledgling would recruit back into the breeding population increased not only with social male age but also with its survival to the following year. This suggests a good‐genes effect may be present for overwintering survival, further arguing for the importance of breeding with old males with proven ability to survive. Thus, the overall reproductive performance of females is clearly boosted by avoiding first‐year breeding males as social mates because selective disappearance ensures a generally higher quality of older males.
Although reproductive performance often increases with paternal age in birds (Forslund and Pärt 1995 ; Rebke et al. 2010 ; Torres et al. 2011 ; Auld et al. 2013 ; Zhang et al. 2015 ), few studies have separated the role of increased experience from selective disappearance of low‐performing males, and only in long‐lived birds. Selective disappearance contributed to the increased reproductive performance with age in blue‐footed booby (Torres et al. 2011 ) and mute swans (Auld et al. 2013 ), but had only a small effect in the common tern (Rebke et al. 2010 ; Zhang et al. 2015 ). For short‐lived birds, the role of selective disappearance has not been investigated in relation to male performance, but selective disappearance of low‐quality females is important in great tits (Bouwhuis et al. 2009 ) and collared flycatchers (Evans et al. 2011 ). There is thus a need for further studies aimed at separating the role of selective disappearance and increased experience for male reproductive performance, especially in short‐lived species.
We did not observe a decline in the reproductive performance of very old males (Figs. 2 and 3 ) as would have been expected based on reproductive senescence and a general decline in phenotypic performance and the ability to provide resources at very old ages (Fig. 1 ). This result contrasts against the observed strong decline in the reproductive performance of female collared flycatchers late in life (Gustafsson and Pärt 1990 ) and with evidence for senescence from other natural populations (Bonduriansky and Brassil 2002 ; Nussey et al. 2013 ; Bouwhuis and Vedder 2017 ; Froy et al. 2019 ; Gaillard and Lemaître 2020 ). Most of the previous scientific works have focused on females making it premature to make the conclusion that actuarial senescence is generally more evident in females even if this indeed appears to be the case in collared flycatchers. However, it has been suggested that sex‐specific reproductive strategies may result in sexual selection favoring males that invest heavily in keeping a competitive phenotype in shape also at old ages and perhaps even at the expense of their germline maintenance (Lemaître and Gaillard 2017 ). Effects of germline senescence are difficult to assess, because genetic effects generally are masked by direct effects of the breeding environment in natural populations (but see below).
Secondly, we assessed the indirect effect of paternal age. Because selective removal of poor‐quality 1‐year‐old individuals resulted in increased reproductive success, we expected that the females would also get genetic benefits from choosing old males. However, there may also be a genetic cost associated with choosing old males due to germline senescence (Fig. 1B ). To separate direct and genetic effects, we used naturally occurring extra‐pair paternity to investigate the relative difference in mass between WPY and EPY within a clutch. This approach has previously been used to investigate if females gain genetic benefits from extra‐pair mating (e.g., Kempenaers et al. 1992 ; Foerster et al. 2003 ; Charmantier et al. 2004 ), but has to our knowledge never been used to investigate effects of male germline senescence. The shared nest environment means that both EPY and WPY benefit from being raised by old males. Because most males in the population are young (Fig. S1 ), but middle‐aged males are most likely to get extra‐pair paternity (Dickinson 2001 ; Bouwman et al. 2007 ; Cleasby and Nakagawa 2012 ; Michálková et al. 2019 ), we expect that EPY in nest with young males as social partners are sired by older males, whereas EPY in nests with very old males as social partners are sired by relatively younger, competitive middle‐aged males, because very old males are rare in the population. The half‐siblings (i.e., within‐ and between‐pair young) sharing the same nest are exposed to the same direct effects of paternal age (Fig. 1C ), which in the flycatcher case lack evidence for a negative effect of actuarial male senescence in broods raised by very old males (Figs. 2 and 3 ). If females gain genetic benefits by mating with old males that have proven their ability to survive (Fig. 1A ), we expect that EPY in nests attended by young males should be of better condition (have higher mass) than WPY (Kempenaers et al. 1992 ; Fig. 1D ). For nests attended by very old males, we also expected EPY to be of better condition (have higher mass), but in this case due to germline deterioration among these very old social males (Fig. 1D ). As expected, EPY indeed outperformed WPY in nests attended by very old males. Surprisingly, however, we found evidence for negative performance effects associated with paternal age also in nests attended by young males where within‐pair offspring outperformed EPY that probably had been sired by “middle‐aged” competitive males. These results imply that the germline of these competitive males may deteriorate before other signs of senescence become obvious. This finding is consistent with the idea that sexual selection has led to the evolution of males that prioritize somatic maintenance at the expense of their germline maintenance (Lemaître and Gaillard 2017 ). One should, however, note that the negative effect was stronger for nests attended by 2‐year‐old rather than 1‐year‐old males. A possible explanation to this finding is that that the two sources of genetic benefits (i.e., avoidance of the effects of germline deterioration by having offspring sired by young males vs. ensuring the effects of good genes by having offspring sired by old males with proven ability to survive) even each other out in nests attended by 1‐year‐old males.
Because mating outside the social pair bond is associated with obvious costs to females, such as exposure to sexually transmitted disease (Sheldon 1993 ) or reduced parental care by the social mate (Arnqvist and Kirkpatrick 2005 ; Griffin et al. 2013 ), much previous scientific work has been allocated toward finding possible adaptive explanations to the wide spread occurrence of active female extra‐pair mating in birds. The most commonly proposed adaptive explanation is that females, who generally are constraint in their choice of social male, can obtain genetic benefits from extra‐pair mating (e.g., Jennions and Petrie 2000 ; Westneat and Stewart 2003 ; Akçay and Roughgarden 2007 ). That old males, with proven ability to survive and more attractive traits, often gain more extra‐pair matings as compared to other males is generally interpreted as evidence in support of this assumption (Akçay and Roughgarden 2007 ; Cleasby and Nakagawa 2012 ). However, this interpretation has recently become questioned (Brooks and Kemp 2001 ; Griffith et al. 2002 ; Beck and Promislow 2007 ; Dean et al. 2010 ; Lifjeld et al. 2011 ; Dupont et al. 2018 ; Rodríguez‐Muñoz et al. 2019 ) and some studies have even found evidence suggesting that EPY may be of lower genetic quality as compared to WPY (Sardell et al. 2011 ; Hsu et al. 2014 ). Thus, the overall evidence for large genetic benefits of extra‐pair mating is limited (Forstmeier et al. 2014 ). Our study implies that there may be negative genetic consequences of extra‐pair mating with old males. Specifically, our results are consistent with a deteriorating germline in old males. There are several mechanisms that can underlie deteriorating of the germline. Mutation accumulation is thought to be a major cause (Kong et al. 2012 ), but possibly also telomere shortening (Noguera et al. 2018 ) as well as passive epigenetic effects (Schroeder et al. 2015 ). We did not investigate the mechanism, but note that they all are evolutionary very similar, resulting from accumulation of deleterious effects with age because of weakened selection (Hamilton 1966 ).
Negative parental age effects are seen as a hallmark of ageing, and several laboratory studies have found that offspring of old parents have shorter life span (Lansing 1947 ; Rockstein 1957 ; Tracey 1958 ; O'Brian 1961 ; Kiritani and Kimura 1967 ; Gavrilov and Gavrilova 1997 ; Priest et al. 2002 ; García‐Palomares et al. 2009 ; Lind et al. 2015 ) and/or reduced fecundity (Priest et al. 2002 ; García‐Palomares et al. 2009 ). Although most often studied in females, negative paternal age effects have also been found in males of captive zebra finch (Noguera et al. 2018 ), Drosophila (Priest et al. 2002 ), and mice (García‐Palomares et al. 2009 ). However, although negative paternal age effects are found in the lab, they are much harder to detect in nature, where they can be masked by positive direct effects of increasing paternal age. Moreover, even negative effects on reproduction with age could reflect somatic ageing and diminished direct effects (Newton and Rothery 2002 ) and hide indirect (genetic) effects. To our knowledge, only one previous study has investigated genetic paternal age effect in wild birds, and found reduced lifetime reproduction of male offspring from old fathers in the house sparrow (Schroeder et al. 2015 ). The study on house sparrows did not consider the relative effects of direct (material) and indirect (genetic) parental age effects on offspring performance, which is the aim of our study. Instead, it focused on paternal genetic effects by using a cross‐foster design and did not investigate the effect of paternal genetic contribution to offspring performance against the same maternal genetic background. By using naturally occurring extra‐pair offspring, we can place our findings in relation to direct benefits from mating with older males and we are testing paternal genetic effects against the same maternal genetic background (i.e., by comparing halfsiblings). Although we found that within‐pair offspring of old social males are smaller than extra‐pair offspring in the same brood, WPY produced by these old males actually have a similar fledging weight as compared to WPY produced by younger males. This finding suggests that old males compensate the disadvantages of the deteriorated germline with increased parental investment. Because the reproductive success of males is very much determined by the ability to attract a mate (Andersson 1994 ), males are expected to invest heavily in secondary sexual characters, perhaps even at the expense of their germline maintenance (Lemaître and Gaillard 2017 ). Thus, males should prioritize keeping their soma in shape. In line with this, male ornamentation in collared flycatchers shows no signs of senescence, but is rather increasing with age (Evans et al. 2011 ), and Houbara bustards that invest considerably in sexual display also show rapid senescence of spermatogenic function (Preston et al. 2011 ). If males prioritize keeping their soma in shape, females may prefer old males to ensure direct benefits in terms of high‐quality territories and parental care.
Our findings also reveal that the ideal situation for a nestling is to be sired by a young male but to be raised in the nest attended by an old male. As a result, despite evident germline ageing, females should prefer old males as social partners because of the direct benefits but obtain extra‐pair mating with younger males. However, older males are generally dominant in aggressive interactions with other males (Andersson 1994 ) suggesting that young males may be prevented from successfully courting and mating with females socially paired with older males. Moreover, young males often arrive later in the season at the breeding grounds as compared to old males reducing the window when they may seek extra‐pair mating before the females lay all their eggs (Canal et al. 2012 ; Edme et al. 2016 ). Finally, males may respond to perceived lost paternity by depressed paternal investment, for example, in terms of reduced paternal care of offspring by their social mate, resulting in selection against EPC behavior in females (Arnqvist and Kirkpatrick 2005 ). Thus, both male‐male interactions and social males’ responses to perceived lost paternity are likely to constrain optimal female mating strategies in relation to male age.
To conclude, we used a combination of long‐term breeding data and naturally occurring EPY to disentangle the different aspects of male age on female fitness in a natural population of collared flycatchers. We found that females paired with older males experienced an overall reproductive advantage due to increased phenotypic quality of males belonging to the older age categories. Selective disappearance of low‐quality males was found to be a major driver behind the advantage of choosing old males as breeding partners. There was moreover evidence for a good gene benefit of choosing old males because males who survived were also more likely to have offspring recruiting back to the breeding population. However, we also found evidence for a negative genetic effect of mating with old males. Within the same nest, offspring sired by old males were of relatively lower quality as compared to their EPY half‐siblings, whereas the opposite was found in nests attended by young males. Direct and indirect benefits from pairing with old males hence act in opposing directions. Female flycatchers gain direct benefits from pairing with old males but genetic benefits from having young males siring their offspring.
Supporting information
Figure S1. Age structure of the population since 2002 until 2018.
Figure S2. Age structure of the population per years since (A) 2008 until (I) 2016.
Table S1. A priori structure of the main models.
Table S2. Age structure total known age individuals monitored between 2002 and 2018.
Table S3. Linear mixed‐effect model (Gaussian) with fledgling number as response variable (cross‐sectional dataset).
Table S4. Generalized mixed‐effect model (genpois) with fledgling number as response variable (cross‐sectional dataset).
Table S5. Linear mixed‐effect model (Gaussian) with number of recruits as response variable (cross‐sectional dataset).
Table S6. Generalized mixed‐effect model (genpois) with number of recruits as response variable (cross‐sectional dataset).
Table S7. Linear mixed‐effect model with fledgling number as response variable (longitudinal dataset).
Table S8. Generalized mixed‐effect model (genpois) with fledgling number as response variable (longitudinal dataset).
Table S9. Linear mixed‐effect model (Gaussian) with number of recruits as response variable of longitudinal dataset (not including birds that died in year 1).
Table S10. Generalized mixed‐effect model (genpois) with number of recruits as response variable of longitudinal dataset (not including birds that died in year 1).
Table S11. Generalized mixed‐effect model (binomial) with recruitment as a response variable.
Table S 12. Generalized mixed‐effect model with binomial distribution for the response variable Recruitment (yes, no) with male's age and male's survival as explanatory variables.
Table S 13. Generalized mixed‐effect model with binomial distribution for the response variable Recruitment (yes, no) with male's age and male's survival as explanatory variables.
Acknowledgments
We thank all the people involved in the fieldwork of the Qvarnström lab flycatcher project for helping with the data collection, C. Cunha for help with Figure 1, and R. Dufva for performing the molecular lab work. We especially thank the editor, associate editor, and two anonymous reviewers for constructive and insightful comments, which greatly improved the manuscript. This work was supported by the Swedish Research Council (Grant number 2016–05138 and 2012–03722 to AQ, grant number 2016–05195 to MIL) and the Stiftelsen för Zoologisk Forskning (to JCS).
Author Contributions
AQ conceived the study. AQ and JCS collected data. JCS performed paternal and all statistical analysis. All authors contributed to the analytical approach and interpretation of the data. All authors wrote the manuscript and approved the final version.
Data Archiving
The data supporting the results are available in the public repository Dryad. DOI: https://doi.org/10.5061/dryad.f1vhhmgxg .
All animal monitoring and handling were in accordance with Swedish laws, following the ethical standards of Linköping's committee of ethical animal research (Linköpings djurförsöketiska nämnd).
LITERATURE CITED
- Akçay, E., and Roughgarden J.. 2007. Extra‐pair paternity in birds: review of the genetic benefits. Evol. Ecol. Res. 9:855–868. https://repository.upenn.edu/biology_papers/12 [ Google Scholar ]
- Alatalo, R. V., Gustafsson L., and Lundberg A.. 1986. Do females prefer older males in polygynous bird species? Am. Nat. 127:241–245. [ Google Scholar ]
- Alund, M., Immler S., Rice A. M., and Qvarnstrom A.. 2013. Low fertility of wild hybrid male flycatchers despite recent divergence. Biol. Lett. 920130169. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Alund, M., Schmiterlöw S. P., McFarlane S. E., and Qvarnström A.. 2018. Optimal sperm length for high siring success depends on forehead patch size in collared flycatchers. Behav. Ecol. 29:1436–1443. [ Google Scholar ]
- Andersson, M. B.1994. Sexual selection. Princeton Univ. Press, Princeton, NJ. [ Google Scholar ]
- Arnqvist, G., and Kirkpatrick M.. 2005. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. Am. Nat. 165:S26–S37. [ DOI ] [ PubMed ] [ Google Scholar ]
- Auld, J. R., Perrins C. M., and Charmantier A.. 2013. Who wears the pants in a mute swan pair? Deciphering the effects of male and female age and identity on breeding success. J. Anim. Ecol. 82:826–835. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bates, D., Mächler M., Bolker B. M., and Walker S. C.. 2015. Fitting linear mixed‐effects models using lme4. J. Stat. Softw. 67:1–48. [ Google Scholar ]
- Beck, C. W., and Promislow D. E. L.. 2007. Evolution of female preference for younger males. PLoS ONE 2e939. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bonduriansky, R., and Brassil C. E.. 2002. Rapid and costly ageing in wild male flies. Nature 420:377. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bouwhuis, S., and Vedder O.. 2017. Avian escape artists? Patterns, processes and costs of senescence in wild birds. In Shefferson R. P., Jones O. R., & Salguero‐Gomez R. (Eds.), The evolution of senescence in the tree of life (pp. 156–174). Cambridge Univ. Press, Cambridge, U.K. [ Google Scholar ]
- Bouwhuis, S., Sheldon B. C., Verhulst S., and Charmantier A.. 2009. Great tits growing old: selective disappearance and the partitioning of senescence to stages within the breeding cycle. Proc. R. Soc. B Biol. Sci. 276:2769–2777. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bouwman, K. M., van Dijk R. E., Wijmenga J. J., and Komdeur J.. 2007. Older male reed buntings are more successful at gaining extrapair fertilizations. Anim. Behav. 73:15–27. [ Google Scholar ]
- Brengdahl, M. I., Kimber C. M., Elias P., Thompson J., and Friberg U.. 2020. Deleterious mutations show increasing negative effects with age in Drosophila melanogaster. BMC Biol. 18:128. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brooks, M. E., Kristensen K., van Benthem K. J., Magnusson A., Berg C. W., Nielsen A., et al. 2017. glmmTMB balances speed and flexibility among packages for zero‐inflated generalized linear mixed modeling. R J. 9:378–400. [ Google Scholar ]
- Brooks, M. E., Kristensen K., Darrigo M. R., Rubim P., Uriarte M., Bruna E., et al. 2019. Statistical modeling of patterns in annual reproductive rates. Ecology 100 e02706. [ DOI ] [ PubMed ] [ Google Scholar ]
- Brooks, R., and Kemp D. J.. 2001. Can older males deliver the good genes? Trends Ecol. Evol. 16:308–313. [ DOI ] [ PubMed ] [ Google Scholar ]
- Canal, D., Jovani R., and Potti J.. 2012. Male decisions or female accessibility? Spatiotemporal patterns of extra pair paternity in a songbird. Behav. Ecol. 23:1146–1153. [ Google Scholar ]
- Charmantier, A., Blondel J., Perret P., and Lambrechts M. M.. 2004. Do extra‐pair paternities provide genetic benefits for female blue tits Parus caeruleus ? J. Avian Biol. 35:524–532. [ Google Scholar ]
- Cleasby, I. R., and Nakagawa S.. 2012. The influence of male age on within‐pair and extra‐pair paternity in passerines. Ibis 154:318–324. [ Google Scholar ]
- Clutton‐Brock, T. H.1984. Reproductive effort and terminal investment in iteroparous animals. Am. Nat. 123:212–229. [ Google Scholar ]
- Comfort, A.2011. The biology of senescence. Rinehart, New York. [ Google Scholar ]
- Conner, J.1989. Older males have higher insemination success in a beetle. Anim. Behav. 38:503–509. [ Google Scholar ]
- Côté, I. M., and Hunte W.. 1993. Female redlip blennies prefer older males. Anim. Behav. 46:203–205. [ Google Scholar ]
- Dean, R., Cornwallis C. K., Løvlie H., Worley K., Richardson D. S., and Pizzari T.. 2010. Male reproductive senescence causes potential for sexual conflict over mating. Curr. Biol. 20:1192–1196. [ DOI ] [ PubMed ] [ Google Scholar ]
- Dickinson, J.2001. Extrapair copulations in western bluebirds ( Sialia mexicana ): female receptivity favors older males. Behav. Ecol. Sociobiol. 50:423–429. [ Google Scholar ]
- Dupont, S. M., Barbraud C., Chastel O., Delord K., Ruault S., Weimerskirch H., et al. 2018. Young parents produce offspring with short telomeres: a study in a long‐lived bird, the Black‐browed Albatross ( Thalassarche melanophrys ). PLoS ONE 13 e0193526. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Edme, A., Munclinger P., and Krist M.. 2016. Female collared flycatchers choose neighbouring and older extra‐pair partners from the pool of males around their nests. J. Avian Biol. 47:552–562. [ Google Scholar ]
- Evans, S. R., Gustafsson L., and Sheldon B. C.. 2011. Divergent patterns of age‐dependence in ornamental and reproductive traits in the collared flycatcher. Evolution 65:1623–1636. [ DOI ] [ PubMed ] [ Google Scholar ]
- Finch, C. E.1998. Variations in senescence and longevity include the possibility of negligible senescence. J. Gerontol. A Biol. Sci. Med. Sci. 53:B235–B239. [ DOI ] [ PubMed ] [ Google Scholar ]
- Foerster, K., Delhey K., Johnsen A., Lifjeld J. T., and Kempenaers B.. 2003. Females increase offspring heterozygosity and fitness through extra‐pair matings. Nature 425:714–717. [ DOI ] [ PubMed ] [ Google Scholar ]
- Forslund, P., and Pärt T.. 1995. Age and reproduction in birds — hypotheses and tests. Trends Ecol. Evol. 10:374–378. [ DOI ] [ PubMed ] [ Google Scholar ]
- Forstmeier, W., Nakagawa S., Griffith S. C., and Kempenaers B.. 2014. Female extra‐pair mating: adaptation or genetic constraint? Trends Ecol. Evol. 29:456–464. [ DOI ] [ PubMed ] [ Google Scholar ]
- Froy, H., Sparks A. M., Watt K., Sinclair R., Bach F., Pilkington J. G., et al. 2019. Senescence in immunity against helminth parasites predicts adult mortality in a wild mammal. Science 365:1296–1298. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gaillard, J. M., and Lemaître J. F.. 2020. An integrative view of senescence in nature. Funct. Ecol. 34:4–16. [ Google Scholar ]
- García‐Palomares, S., Navarro S., Pertusa J. F., Hermenegildo C., García‐Pérez M. A., Rausell F., et al. 2009. Delayed fatherhood in mice decreases reproductive fitness and longevity of offspring. Biol. Reprod. 80:343–349. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gavrilov, L. A., and Gavrilova N. S.. 1997. Parental age at conception and offspring longevity. Rev. Clin. Gerontol. 7:5–12. [ Google Scholar ]
- Grant, B. R., and Grant P. R.. 1987. Mate choice in Darwin's finches. Biol. J. Linn. Soc. 32:247–270. [ Google Scholar ]
- Griffin, A. S., Alonzo S. H., and Cornwallis C. K.. 2013. Why do cuckolded males provide paternal care? PLoS Biol. 11e1001520. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Griffith, S. C., Owens I. P. F., and Thuman K. A.. 2002. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 11:2195–2212. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gustafsson, L., and Pärt T.. 1990. Acceleration of senescence in the collared flycatcher Ficedula albicollis by reproductive costs. Nature 347( 6290 ):279–281. 10.1038/347279a0 [ DOI ] [ Google Scholar ]
- Hamilton, W. D.1966. The moulding of senescence by natural selection. J. Theor. Biol. 12:12–45. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hsu, Y. H., Schroeder J., Winney I., Burke T., and Nakagawa S.. 2014. Costly infidelity: low lifetime fitness of extra‐pair offspring in a passerine bird. Evolution 68:2873–2884. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jennions, M. D., and Petrie M.. 2000. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 75:21–64. [ DOI ] [ PubMed ] [ Google Scholar ]
- Jones, A. G., Small C. M., Paczolt K. A., and Ratterman N. L.. 2010. A practical guide to methods of parentage analysis. In Molecular Ecology Resources 10(1):6–30. 10.1111/j.1755-0998.2009.02778.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Kalinowski, S. T., Taper M. L., and Marshall T. C.. 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16:1099–1106. [ DOI ] [ PubMed ] [ Google Scholar ]
- Källander, H., and Smith H. G.. 1990. Manipulation of the brood size of pied flycatchers. Pp. 257–268 in Blondel J., Gosler A., Lebreton J. D., and McCleery R., eds. Population biology of passerine birds. Springer, Berlin. [ Google Scholar ]
- Kemkes‐Grottenthaler, A.2004. Parental effects on offspring longevity ‐ evidence from 17th to 19th century reproductive histories. Ann. Hum. Biol. 31:139–158. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kempenaers, B., and Dhondt A.. 1993. Why do females engage in extra‐pair copulations? A review of hypotheses and their predictions. Belgian J. Zool. 123:93–103. [ Google Scholar ]
- Kempenaers, B., and Sheldon B. C.. 1996. Why do male birds not discriminate between their own and extra‐pair offspring? Anim. Behav. 51:1165–1173. [ Google Scholar ]
- Kempenaers, B., Verheyen G. R., Van Den Broeck M., Burke T., Van Broeckhoven C., and Dhondt A.. 1992. Extra‐pair paternity results from female preference for high‐quality males in the blue tit. Nature 357:494–496. [ Google Scholar ]
- Kiritani, K., and Kimura K.. 1967. Effects of parental age on the life cycle of the southern green stink bug, Nezara viridula L. (Heteroptera: Pentatomidae). Appl. Entomol. Zool. 2:69–78. [ Google Scholar ]
- Knief, U., and Forstmeier W.. 2020. Violating the normality assumption may be the lesser of two evils. bioRxiv. 10.1101/498931 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kokko, H.1998. Good genes, old age and life‐history trade‐offs. Evol. Ecol. 12:739–750. [ Google Scholar ]
- Kokko, H., and Lindstrom J.. 1996. Evolution of female preference for old mates. Proc. R. Soc. B Biol. Sci. 263:1533–1538. [ Google Scholar ]
- Kong, A., Frigge M. L., Masson G., Besenbacher S., Sulem P., Magnusson G., et al. 2012. Rate of de novo mutations and the importance of father's age to disease risk. Nature 488:471–475. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lansing, A. I.1947. A transmissible, cumulative, and reversible factor in aging. J. Gerontol. 2:228–239. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lemaître, J. F., and Gaillard J. M.. 2017. Reproductive senescence: new perspectives in the wild. Biol. Rev. 92:2182–2199. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lifjeld, J. T., Kleven O., Jacobsen F., McGraw K. J., Safran R. J., and Robertson R. J.. 2011. Age before beauty? Relationships between fertilization success and age‐dependent ornaments in barn swallows. Behav. Ecol. Sociobiol. 65:1687–1697. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lind, M. I., Berg E. C., Alavioon G., and Maklakov A. A.. 2015. Evolution of differential maternal age effects on male and female offspring development and longevity. Funct. Ecol. 29:104–110. [ Google Scholar ]
- Marshall, T. C., Slate J., Kruuk L. E. B., and Pemberton J. M.. 1998. Statistical confidence for likelihood‐based paternity inference in natural populations. Mol. Ecol. 7:639–655. [ DOI ] [ PubMed ] [ Google Scholar ]
- Medawar, P. B.1951. An unsolved problem of biology. H. K. Lewis & Co Ltd, Lond. [ Google Scholar ]
- Michálková, R., Tomášek O., Adámková M., Kreisinger J., and Albrecht T.. 2019. Extra‐pair paternity patterns in European barn swallows Hirundo rustica are best explained by male and female age rather than male ornamentation. Behav. Ecol. Sociobiol. 73:119. [ Google Scholar ]
- Monaghan, P., Maklakov A. A., and Metcalfe N. B.. 2020. Intergenerational transfer of ageing: parental age and offspring lifespan. Trends Ecol. Evol. 35:927–937. [ DOI ] [ PubMed ] [ Google Scholar ]
- Newton, I., and Rothery P.. 2002. Age‐related trends in different aspects of the breeding performance of individual female Eurasian sparrowhawks ( Accipiter nisus ). Auk 119:735–748. [ Google Scholar ]
- Noguera, J. C., Metcalfe N. B., and Monaghan P.. 2018. Experimental demonstration that offspring fathered by old males have shorter telomeres and reduced lifespans. Proc. R. Soc. B Biol. Sci. 28520180268. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nussey, D. H., Froy H., Lemaitre J. F., Gaillard J. M., and Austad S. N.. 2013. Senescence in natural populations of animals: widespread evidence and its implications for bio‐gerontology. Ageing Res. Rev. 12:214–225. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- O'Brian, D. M.1961. Effects of parental age on the life cycle of Drosophila melanogaster . Ann. Entomol. Soc. Am. 54:412–416. [ Google Scholar ]
- Pizzari, T., Dean R., Pacey A., Moore H., and Bonsall M. B.. 2008. The evolutionary ecology of pre‐ and post‐meiotic sperm senescence. Trends Ecol. Evol. 23:131–140. [ DOI ] [ PubMed ] [ Google Scholar ]
- Preston, B. T., Jalme M. S., Hingrat Y., Lacroix F., and Sorci G.. 2011. Sexually extravagant males age more rapidly. Ecol. Lett. 14:1017–1024. [ DOI ] [ PubMed ] [ Google Scholar ]
- Priest, N. K., Mackowiak B., and Promislow D. E. L.. 2002. The role of parental age effects on the evolution of aging. Evolution 56:927–935. [ DOI ] [ PubMed ] [ Google Scholar ]
- Qvarnström, A., Rice A. M., and Ellegren H.. 2010. Speciation in Ficedula flycatchers. Philos. Trans. R. Soc. B Biol. Sci. 365:1841–1852. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- R Core Team . 2019. A language and environment for statistical computing. Available via https://www.r‐project.org/ .
- Rebke, M., Coulson T., Becker P. H., and Vaupel J. W.. 2010. Reproductive improvement and senescence in a long‐lived bird. Proc. Natl. Acad. Sci. USA 107:7841–7846. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rockstein, M.1957. Longevity of male and female house flies. J. Gerontol. 12:253–256. [ DOI ] [ PubMed ] [ Google Scholar ]
- Rodríguez‐Muñoz, R., Hopwood P., Fisher D., Skicko I., Tucker R., Woodcock K., et al. 2019. Older males attract more females but get fewer matings in a wild field cricket. Anim. Behav. 153:1–14. [ Google Scholar ]
- Sardell, R. J., Arcese P., Keller L. F., and Reid J. M.. 2011. Sex‐specific differential survival of extra‐pair and within‐pair offspring in song sparrows, Melospiza melodia . Proc. R. Soc. B Biol. Sci. 278:3251–3259. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schroeder, J., Nakagawa S., Rees M., Mannarelli M. E., and Burke T.. 2015. Reduced fitness in progeny from old parents in a natural population. Proc. Natl. Acad. Sci. USA 112:4021–4025. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shefferson, R. P., Jones O. R., and Salguero‐Gómez R.. 2017. The evolution of senescence in the tree of life. Cambridge Univ. Press, Cambridge, U.K. [ Google Scholar ]
- Sheldon, B. C.1993. Sexually transmitted disease in birds: occurrence and evolutionary significance. Philos. Trans. R. Soc. London, B 339:491–497. [ DOI ] [ PubMed ] [ Google Scholar ]
- Shirihai, H., and Svensson L.. 2018. Handbook of Western palearctic birds, Volume 2 . Passerines: flycatchers to buntings . 1st ed.Bloomsbury Publishing, Lond. [ Google Scholar ]
- Smeds, L., Qvarnström A., and Ellegren H.. 2016. Direct estimate of the rate of germline mutation in a bird. Genome Res. 26:1211–1218. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Takagi, M.2003. Different effects of age on reproductive performance in relation to breeding stage in Bull‐headed Shrikes. J. Ethol. 21:9–14. [ Google Scholar ]
- Torres, R., Drummond H., and Velando A.. 2011. Parental age and lifespan influence offspring recruitment: a long‐term study in a seabird. PLoS ONE 6e27245. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tracey, K. M.1958. Effects of parental age on the life cycle of the mealworm, Tenebrio molitor Linnaeus. Ann. Entomol. Soc. Am. 51:429–432. [ Google Scholar ]
- Trivers, R. L.1972. Parental investment and sexual selection. Pp. 136–179 in Campbell B., ed. Sexual selection and the descent of man, 1871–1971. Aldine, Chicago. [ Google Scholar ]
- Velando, A., Noguera J. C., Drummond H., and Torres R.. 2011. Senescent males carry premutagenic lesions in sperm. J. Evol. Biol. 24:693–697. [ DOI ] [ PubMed ] [ Google Scholar ]
- Weatherhead, P. J.1984. Mate choice in avian polygyny: why do females prefer older males? Am. Nat. 123:873–875. [ Google Scholar ]
- Westneat, D. F., and Stewart I. R. K.. 2003. Extra‐pair paternity in birds: causes, correlates, and conflict. Annu. Rev. Ecol. Evol. Syst. 34:365–396. [ Google Scholar ]
- Wickham, H.2016. ggplot2: elegant graphics for data analysis. Springer‐Verlag, New York. Available via https://ggplot2.tidyverse.org . [ Google Scholar ]
- Zhang, H., Vedder O., Becker P. H., and Bouwhuis S.. 2015. Age‐dependent trait variation: the relative contribution of within‐individual change, selective appearance and disappearance in a long‐lived seabird. J. Anim. Ecol. 84:797–807. [ DOI ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data availability statement.
- View on publisher site
- PDF (575.3 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Find over 25,000 psychological definitions
good genes hypothesis
Browse dictionary by letter, psychology term of the day.
December 24th 2024

IMAGES
COMMENTS
Apr 19, 2018 · a hypothesis of female mate selection arguing that certain features of male behavior and body structure reflect genetic variations that are correlated with positive survival attributes such as health and strength and that females choose males with such features, thereby enhancing their offspring’s chances of survival.
Good genes hypothesis, in biology, an explanation which suggests that the traits females choose when selecting a mate are honest indicators of the male’s ability to pass on genes that will increase the survival or reproductive success of her offspring. Although no completely unambiguous examples
Jan 13, 2023 · The good genes hypothesis (GGH) was formulated by evolutionary biologist W.D. Hamilton and behavioral ecologist M. Zuk ().It proposes that the characteristics preferred by females are a signal of males’ ability to pass on genes (coding that certain characteristic) which will increase the survival and reproductive success of the offspring sired with a male possessing them.
The good genes hypothesis is a model of sexual selection that claims mate preferences for particular traits, e.g. for beauty, have evolved because these traits are reliable indicators of overall health and other 'good' genetic traits, Thus, the theory claims, mate choice is not evolutionarily maladaptive or neutral, but beneficial and eugenic in terms population viability.
The good genes hypothesis is a theory that explains what? a. why more fit individuals are more likely to have more offspring b. why alleles that confer beneficial traits or behaviors are selected for by natural selection c. why some deleterious mutations are maintained in the population d. why individuals of one sex develop impressive ...
May 11, 2013 · good genes hypothesis By N., Sam M.S. Hypothesis of female mate selection that argues genetic variation in males correlates with success, features of male behaviour provide information about variation , females respond to variation by choosing males with good genes.
The hypothesis is often studied in the context of sexual selection and mate choice. Traits indicating 'good genes' can include physical characteristics, behaviors, or other phenotypic expressions. Good genes can confer advantages such as disease resistance, better metabolism, or more efficient resource use.
Aug 24, 2021 · The “good genes” hypothesis predicts a preference for older individuals based on the logic that survival until older ages requires high‐quality genes, which in turn will be passed on to the offspring (Trivers 1972; Kokko and Lindstrom 1996; Kokko 1998; Bouwman et al. 2007).
In the "good genes" hypothesis, ornaments may indicate that males are any of the following EXCEPT: able to provide a direct benefit to females All of the following supports sexual conflict as an explanation for Brennan's findings on the reproductive organs of ducks and waterfowl, EXCEPT:
good genes hypothesis a hypothesis of female mate selection arguing that certain features of male behavior and body structure reflect genetic variations that are correlated with positive survival attributes such as health and strength and that females choose males with such features, thereby enhancing their offspring’s chances of survival.