- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar

- FREE Experiments
- Kitchen Science
- Climate Change
- Egg Experiments
- Fairy Tale Science
- Edible Science
- Human Health
- Inspirational Women
- Forces and Motion
- Science Fair Projects
- STEM Challenges
- Science Sparks Books
- Contact Science Sparks
- Science Resources for Home and School

How to make Ice Cream in a Bag
September 6, 2018 By Emma Vanstone 6 Comments
Did you know you can make homemade ice cream in a bag in less than 10 minutes with just milk, salt and ice? You don’t even need a freezer!! Our ice cream recipe is easy for kids to follow and a fun science activity at the same time.
Experiment with different flavours and toppings to find your favourite ice cream dessert!
This awesome kitchen science experiment for kids involves lots of interesting chemistry, is inexpensive and very simple!
What is ice cream?
Ice cream is made up of droplets of fat from milk jumbled up with millions of tiny crystals of ice and pockets of air.
This activity uses the freezing power of salt and ice to create ice crystals in milk without a freezer!
Homemade Ice Cream in a Bag Science Experiment
What you need to make ice cream in a bag.
A large bag of ice
Milk – we used chocolate milk, but any kind of milk or non-dairy drink will work
A tablespoon of sugar and a teaspoon of vanilla essence – optional
Resealable bags

How to make ice cream in a bag
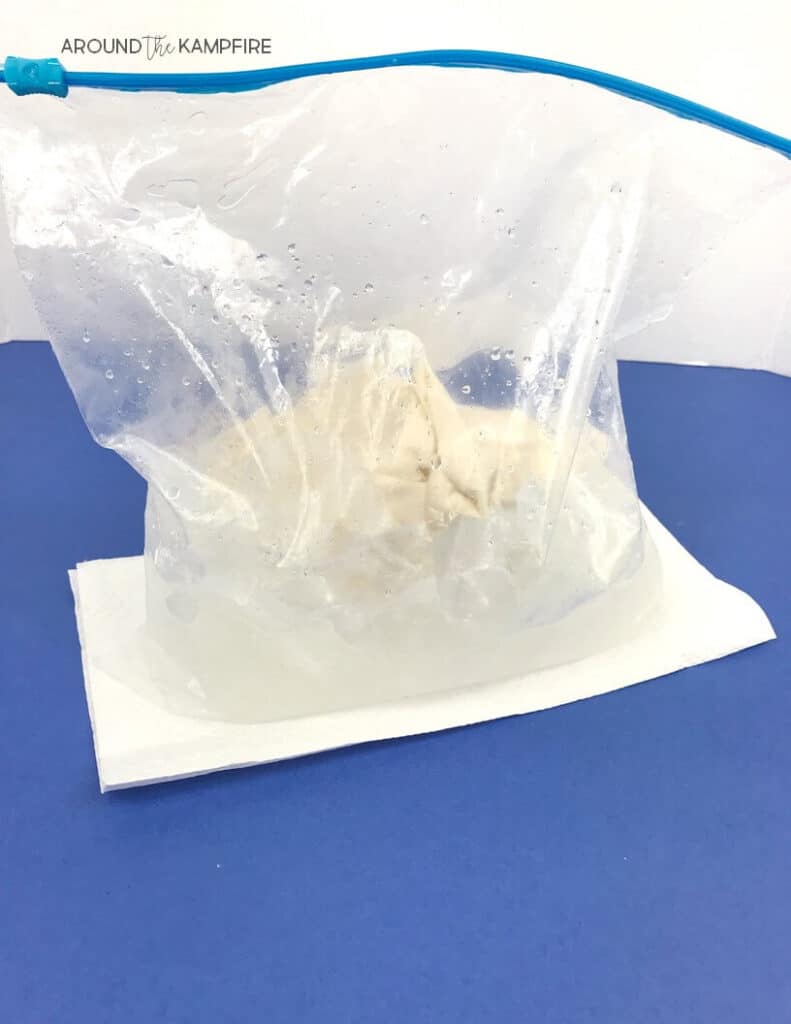
- Pour a cup of milk into a resealable plastic bag. Add the sugar and vanilla essence ( or just use flavoured milk ). Make sure the bag is properly sealed.
- Half-fill a bigger sealable bag with ice and add a good amount of salt.
- Put the milk bag into the ice bag and give it a good shake.
- Keep the milk in contact with the ice as much as possible.
- Keep rolling the ice over the milk. The ice in the bag will get VERY cold, so put a towel around the bag to protect your hands.
- Check the milk after 5 minutes. It should be a similar consistency to ice cream. If not, keep going for a bit longer.

Why does ice cream in a bag work?
Pure water freezes at 0°C. The addition of salt lowers the freezing point by a few degrees ( freezing point depression ). This means when salt is added to the ice in the outer bag, the ice (which is at 0°C) is above its freezing point, so it starts to melt. Melting needs energy which in this case comes from the milk mixture in the inner bag. Heat energy is absorbed from the milk making ice crystals form between the tiny fat molecules.
The more salt that is added to the ice, the lower the freezing point. For the ice to melt, heat must be absorbed from the surroundings ( in this case, the milk mixture ), causing it to freeze.
The ice will feel VERY cold, which is why you’ll need a towel to cover the bag after a few minutes.
Practical applications of salt – why is salt added to roads in cold weather?
During cold weather, salt and grit are applied to roads, the salt makes the ice melt even if the air temperature is below freezing point.
Extension ideas
Create and test different homemade ice cream recipes.
Place a mixture of ice and salt in a freezer to investigate whether it freezes or not.
This activity would be perfect for a science club or a fun STEM Challenge . You could even have a competition to see who can make the COLDEST ice cream!

More Awesome Kitchen Science for Kids
Make a whole meal of science experiments ! This is a great science or cooking challenge for homeschooling or school!
Try one of my other easy kitchen science experiments for kids , including finding out why cakes turn brown in the oven, why pineapple stops jelly setting, building towers with toothpicks in a flapjack or brownie base and lots more!
If your children love edible experiments, you might also like my kitchen science book, Snackable Science which includes SIXTY fun and easy edible experiments !

Affiliate links

Last Updated on July 1, 2023 by Emma Vanstone
Safety Notice
Science Sparks ( Wild Sparks Enterprises Ltd ) are not liable for the actions of activity of any person who uses the information in this resource or in any of the suggested further resources. Science Sparks assume no liability with regard to injuries or damage to property that may occur as a result of using the information and carrying out the practical activities contained in this resource or in any of the suggested further resources.
These activities are designed to be carried out by children working with a parent, guardian or other appropriate adult. The adult involved is fully responsible for ensuring that the activities are carried out safely.
Reader Interactions
August 16, 2015 at 6:35 am
ooh we are going to have to try that Emma
August 16, 2015 at 11:46 am
Great science activity, thanks for sharing. I think I will try this with my kids this week, they’ll love it!
February 12, 2019 at 10:52 am
Great activity, I Love winter season so much.Reading your article I have found some awesome tips. Thanks for sharing this nice post with us.
February 05, 2022 at 4:47 pm
My kids and I just did this with snow because, well, why nit? It was fun shaking the bag! And we all tasted the 4 flavors we made. Very easy and cool pun intended). Thanks!
June 28, 2023 at 1:23 pm
would it work with a dairy alternative such as rice milk or coconut milk?
June 29, 2023 at 11:03 am
yes, definitely
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
How to Make Ice Cream in a Bag – Tasty Science Project

Making ice cream in a bag is a fun, tasty science project that both kids and adults enjoy. It’s not just an innovative way to whip up a delicious dessert—it’s also an educational experience that demonstrates fascinating concepts of science. The beauty of this project is that it doesn’t require a freezer or an ice cream machine. All you need are some everyday ingredients and materials, a love for ice cream, and a touch of curiosity about the world of science!
Ice Cream in a Bag Materials
Here’s a list of ingredients and materials you’ll need to make homemade ice cream in a bag:
Ingredients:
- 1 cup half-and-half (or milk for a lighter version)
- 2 tablespoons granulated sugar
- 1/2 teaspoon pure vanilla extract
- Mix-ins like chocolate chips, fruit, or cookie crumbs (optional)
For a vegan variation, you can use a non-dairy milk alternative like almond, soy, or oat milk. Whatever milk you choose, a product with a higher fat content produces a creamier ice cream. You might get ice crystals if you use low-fat or skim milk.
For the Ice-Salt Mixture:
- 1/3 cup rock salt or kosher salt (we’ll talk more about why we use these specific types of salt later)
The quantities of ice and salt are not critical and you don’t need to measure them. Basically, you fill the bag with ice and sprinkle on salt. Rock salt or kosher salt are ideal for this project because they have a larger grain size, so they mix well with ice rather than just sinking to the bottom of the bag. But, if regular table salt is what you have, it works just fine.
- 1 small zip-top bag (about a pint/quart size)
- 1 large zip-top bag (about a gallon size)
- Gloves or a towel to protect hands from the cold
Let’s Make Ice Cream!
Here’s how you can create your very own ice cream in a bag:
- In the smaller bag, combine the half-and-half (or milk), sugar, and vanilla extract. Squeeze out excess air and seal the bag tightly.
- Fill the larger bag halfway with ice, then sprinkle the rock or kosher salt over it.
- Place the sealed smaller bag into the larger bag with the ice-salt mixture.
- Seal the larger bag. If you’re using gloves, put them on now. If you’re using a towel, wrap it around the bag.
- Shake the bag vigorously for about 5-10 minutes, or until the mixture in the smaller bag hardens to a consistency similar to that of soft-serve ice cream.
- Carefully remove the smaller bag, being sure to wipe off any salt before opening it.
- At this point, add in any mix-ins you like, or even flavor your ice cream with chocolate or fruit puree.
The final product has a creamy, soft-serve consistency and a deliciously simple vanilla flavor that serves as a great canvas for various mix-ins and flavor variations.
The Science Behind It
This fun and tasty project showcases an important scientific concept called freezing point depression . When you add salt to the ice, it lowers the freezing point of water , which is normally 0°C or 32°F, down as low as -21 °C or -5 °F.
How does this work? Salt is sodium chloride (NaCl), which separates into sodium (Na + ) and chlorine Cl – ) ions. These ions interfere with water molecules getting close enough together for hydrogen bonding, which play a significant role in freezing.
So, as the ice melts, it dissolves some of the salt. Dissolving salt in water is an endothermic reaction that absorbs heat from the environment, which includes the ice cream mixture. The ice cream mixture in the bag actually freezes faster than it would in a regular freezer.
In this process, the type of salt matters. Salts that break into more than two ions lower the freezing point of water more than sodium chloride does. For example, calcium chloride (CaCl 2 ) breaks into one calcium ion and two chlorine ions and lower the freezing point down to -29 °C or -20 °F. Sugar and other covalent compounds also dissolve in water and lower the freezing point. But, since they only dissolve into molecules rather than ions, their effect is not as significant.
Further Experiments
There’s always more to discover with this project! You can experiment with different types of salts—like table salt, sea salt, or even Epsom salt—to observe their effects on the freezing process. You might find differences in how quickly the ice cream forms, or how smooth the final product is. Also, explore variations in the recipe, such as adding chocolate or other flavorings to the mixture. Enjoy the exploration and have fun experimenting!
- Atkins, Peter (2006). Atkins’ Physical Chemistry . Oxford University Press. ISBN 0198700725.
- Pedersen, U.R.; et al. (August 2016). “Thermodynamics of freezing and melting”. Nature Communications . 7 (1): 12386. doi: 10.1038/ncomms12386
- Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002). General Chemistry (8th ed.). Prentice-Hall. ISBN 0-13-014329-4.
Related Posts
Get Your ALL ACCESS Shop Pass here →

Easy Homemade Ice Cream In a Bag
Yes, making homemade ice cream in a bag does work! Whether you make it inside or outdoors, have a pair of warm gloves ready. This homemade ice cream science experiment is chilly chemistry for kids you can eat! Enjoy fun science experiments all year round!

Ice Cream in a Bag Recipe
Making homemade ice cream in a bag is easy and a good arm workout! This ice cream in a bag science experiment is a fun edible science activity to try at home or in the classroom. It does require some adult supervision and assistance. A good pair of gloves is needed, as this science activity gets very cold.
💡 ALSO CHECK OUT: More Ice Cream Activities for Kids
Ingredients:
- Free printable recipe below
- 1/2 cup half and half (cream and milk)
- ¼ tsp vanilla
- 1 tbsp sugar
- ⅓ cup kosher or rock salt
- Gallon size zip-top bag(s)
- Quart size zip-top bag(s)
- Sprinkles, chocolate sauce, fruit (optional but really “the best part” ingredients!)
How to Make Ice Cream in A Bag
Watch the video:.
STEP 1. Place the ice and salt in a gallon-size bag; set aside.
STEP 2. In a smaller bag, mix half and half of the vanilla and sugar. Make sure to seal the bag tightly.

STEP 3. Place the smaller bag inside the gallon size bag. Shake the bags for about 5 minutes until your milk is solid. Make sure to use gloves as the bag gets very cold.
💡 TIP: If you find your ice cream in a bag does not work, try it with more ice cubes and salt and shake for 5 more minutes.

Time to enjoy your yummy homemade ice cream! Store any uneaten ice cream in the zip-top bag. Place it in the freezer and enjoy for next time!

Ice Cream Science
What’s the chemistry behind ice cream in a bag? It’s pretty sweet! The magic is in the salt and ice mixture in the bag!
To make homemade ice cream, your ingredients must get very cold and freeze. Instead of placing the ingredients for ice cream in the freezer, you mix salt and ice to make a solution.
Adding salt to the ice lowers the temperature at which water freezes. You will notice your ice melting as your ice cream ingredients start to freeze. You can also see this with our ice melting experiments .
Shaking the bag allows the warm cream mixture to move around, allowing for better freezing. It also creates some air, making the ice cream a bit fluffier.
💡 Is ice cream a liquid or a solid? Homemade ice cream changes states of matter .
It starts as a liquid but changes to a solid in its frozen form, but it can go back to a liquid when it melts. This is a good example of reversible change, as it’s not permanent.
You will notice that the bag becomes much too cold to handle without gloves, so please make sure you have a good pair of gloves to shake it with.

Turn It Into An Ice Cream In A Bag Experiment
If you want to make this truly a science experiment using the scientific method , you need to change one variable. Read more about the scientific method for kids below.
Take this easy ice cream in a bag recipe and turn it into a science project with one of these suggestions:
- What happens if you don’t use salt? Set up two bags for making ice cream, but leave the salt out of one bag.
- What happens if you use a different type of salt? Set up two or more bags for making ice cream and choose different types of salt to test!
- What happens if you swap out the milk for the heavy cream? Or what happens if you try another type of milk, like almond milk? Set up two or more bags for making ice cream and choose different types of milk to test!
More Fun Food Experiments
- Shake up some butter in a jar
- Try Strawberry DNA Extraction
- Experiment with Cabbage pH Chemistry
- Make Edible Geodes
- Set Up Fizzing Lemonade
- Make Maple Syrup Snow Candy
- Try this easy sorbet recipe

Printable Science Projects Pack
If you’re looking to grab all of our printable science projects in one convenient place plus exclusive worksheets and bonuses like a STEAM Project pack, our Science Project Pack is what you need! Over 300+ Pages!
- 90+ classic science activities with journal pages, supply lists, set up and process, and science information. NEW! Activity-specific observation pages!
- Best science practices posters and our original science method process folders for extra alternatives!
- Be a Collector activities pack introduces kids to the world of making collections through the eyes of a scientist. What will they collect first?
- Know the Words Science vocabulary pack includes flashcards, crosswords, and word searches that illuminate keywords in the experiments!
- My science journal writing prompts explore what it means to be a scientist!!
- Bonus STEAM Project Pack: Art meets science with doable projects!
- Bonus Quick Grab Packs for Biology, Earth Science, Chemistry, and Physics
- Science Fair Project Pack with experiments to try!

13 Comments
What is “Half and half”? Can you do it without the rock salt, cannot find it here.
We use sweetened condensed milk (1 can) and 2 liters of your favorite soda pop. It makes the most amazing tasting ice cream ever!!
Half and half is a creamer.
We used coarse ground salt.
Did I miss where you mention how much of each ingredient?
There is a link that says check out the recipe. Since It is not my own original recipe, etiquette requires me to link to it. I will highlight the link.
- Pingback: Simple Summer Science Experiments and STEAM Activities
- Pingback: Preschool Science Experiments and Science Activities
- Pingback: 25 Fun Summer Crafts And Activity’s!
- Pingback: 12 Easy Summer Science Experiments – Nature's Workshop!
I love this idea! I would love to use it in a class setting, but am wondering how much each batch makes? Is this recipe for one person or multiple? I mean, we can all use more ice cream, but minimalistically speaking, lol
Hi! We always need more ice cream but I guess it would come down to how much you would expect to serve each kiddo. I think 2-3 kids could enjoy tasting it and surely it would be helpful to have multiple hands to shake each bag since it can get tiring. Make sure to really seal bags well though so that the salt doesn’t make it’s way into the ice cream bag. I have thought about a plastic container with the ingredients and that be put inside the bag with ice and salt…
- Pingback: Grow Sugar Crystals for Edible Rock Candy Chemistry Experiment
Comments are closed.

Subscribe to receive a free 5-Day STEM Challenge Guide
~ projects to try now ~.


The Science Scoop: How to make ice cream in a bag (and learn while you do!)
I’ve got a very tasty science experiment to do today!
Making ice cream in a bag is all science! This exciting experiment allows you to create your own frozen treat while delving into the world of freezing point depression, heat transfer, and the importance of agitation.
So skip the store and make your own delicious ice cream while talking about science!

How to make the Science Scoop homemade ice cream experiment
Supplies you will need.
For this experiment, you’ll need:
- Sugar (1 tablespoon)
- Ice (about 5 cups)
- Half and half (1/2 cup)
- Salt (1/2 cup)
- Vanilla extract (1/4 teaspoon)
For our lactose-intolerant friends: try using full-fat, canned coconut milk in place of the half and half. The fat content of the coconut milk should help it produce similar results.

Before you start
You can use a few variations of this experiment:
- If you’re lactose-intolerant, try using full-fat coconut milk from the can
- Chocolate ice cream is easy; just add cocoa powder
- You can add toppings too, if you have them!
Instructions
Here is how to do this experiment with your child:
Step 1: Make the ice cream bag
First, we are going to add the ingredients to the sealable sandwich bag.
Add 1 tablespoon of sugar, 1/2 cup of half and half, and 1/4 teaspoon of vanilla.
Seal the bag and place it to the side.

Step 2: Fill the gallon-sized bag
Add 4-5 cups of ice and about a 1/2 cup of salt to the gallon-sized bag.


Step 3: Place ice cream bag in gallon-sized bag and shake
Place the sealed sandwich-sized bag in the gallon-sized bag with ice and salt and seal it.
Place a towel over the bag and start shaking!
You will need to shake the bag for about 5-10 minutes. Every few minutes, feel the ice cream bag to see if the consistency has changed.
Step 4: Compare the results
After about a minute, take a look at the ice cream bag. What do you notice? Keep pausing to check out the ice cream bag to see how it changes over the next 5 minutes.
Step 5: Enjoy your science experiment!
What are you waiting for? Enjoy your ice cream!
You can add sprinkles or chocolate chips, place it in a cone, or even add some cocoa powder to make it chocolate ice cream.

The STEM behind the Science Scoop homemade ice cream experiment
This experiment teaches:
Freezing point depression
Heat transfer and air incorporation, how it works.
Making ice cream in a bag is more than just a tasty treat – it’s science!
This experiment is a mix of freezing point depression, heat transfer, and air incorporation. The milk mixture gradually loses heat and transitions into a semi-frozen state. The sugar also helps lower the freezing point of the liquid slightly, which contributes to the overall freezing process.
When it’s done, you have a creamy ice cream in a bag!
This experiment demonstrates the principle of freezing point depression, which means it lowers the temperature at which the ice can exist as a solid.
Normally, water molecules arrange themselves in a specific way at 0°F, forming ice crystals. This arrangement doesn’t require much energy.
When you add salt to the ice, it dissolves into individual ions (sodium and chloride). These ions disrupt the orderly arrangement of water molecules, making it harder for them to form ice crystals.
Since water molecules struggle to form crystals in the presence of salt ions, they need to reach a lower temperature to achieve the same ordered structure. This is why the freezing point of the salt water is lower than that of pure water.
The more salt you add, the lower the freezing point and the faster the ice cream will freeze.
Utilizing the principle of freezing point depression occurs in other areas of life too, like preserving food or antifreeze in car radiators.
Cool , huh?
As the ice in the outer bag melts, it absorbs heat from the inner bag containing the ice cream mixture. This transfer of heat is the driving force behind the freezing process.
The absorbed heat from the ice cream mixture causes its temperature to drop, initiating the formation of ice crystals and gradually transforming the liquid mixture into ice cream. The colder the ice bath (thanks to the salt), the faster this heat exchange occurs.
The second important piece here is the air incorporation, which occurs because we are shaking the bag. The small air pockets that are created from shaking the bag act like separators, preventing the formation of large ice crystals.
It takes several minutes to do this experiment, which will give your child a chance to practice patience (which is especially hard when it comes to ice cream!).
More experiments about food to try out with your child
Build a Solar Oven Snack Shack!
Related experiments
It's time to harness the power of the sun to make delicious treats! Building a solar oven snack shack is a great way to talk to your child about reflectivity (foil), solar absorption (black...
Science Experiments with Ice Cream

Who doesn’t love ice cream? See my list of super fun science experiments with ice cream for your young scientists! These activities are perfect for children and super easy to try at home.
Thinking of fun ways to celebrate summer with tons of learning for your kids? See these fun summer activities for kids .
Every single one of these ice cream-inspired experiments is not just about making a dessert with your little sweet tooth, they can help kids understand the basic principles of chemistry and observation. Plus, they get to see real-life results while having a tasty treat right after the experiment!
Get ready to mix learning and summer desserts!

This post may contain affiliate links meaning I get commissions for purchases made through links in this post. Read my disclosure policy here.
What is the science behind the ice cream experiment?
Have you and your kids ever made ice cream at home? It’s super fun and tasty! But does your little learner know why and how it works?
When you mix ice cream ingredients together like cream, milk, and sugar, you need to make them very cold. We can use ice and salt for this. The salt lowers the melting point of ice, making it colder than usual. This helps freeze the milk, cream, and sugar mixture!
As we shake shake shake the bag, tiny ice crystals form in the mix. These tiny crystals are what give ice cream its smooth texture. All you have to do is to keep shaking to make sure the mixture gets cold and freezes.
This shows how simple mixtures and variance in temperatures can make new things. It also boosts curiosity and problem-solving skills in your child’s young mind.
Lastly, making ice cream at home is a fun way to spend some unforgettable bonding time with friends and family, especially during summer when ice cream is a well-known favorite.
The fun part about these ice cream science activities is your kids get to eat yummy ice cream when you’re done. Enjoy!
Recommended Ice Cream Books
Discover a world of delightful tales and sweet adventures with my curated list of recommended ice cream books for kids. These engaging stories will not only entertain but also inspire a love for reading in young minds.

Recommended Ice Cream Educational Toys
Get ready for a delightful learning adventure with our list of educational ice cream-themed toys for kids! These playful yet informative toys make learning fun, combining the joy of sweet treats with essential educational concepts.

Fun Summer Activities for Kids
Are you thinking of a way to make your child’s summer extra special this year? Check out these fun summer activities! A combination of having fun and learning all rolled into one.
- Jello Play Dough Recipe: Seashell theme
- How to Make Super Bubbles
- Celebration Slime Recipe
- Early Writing Ideas with Sidewalk Chalk
More Ice Cream Activities
Don’t let the opportunity to do more ice cream activities with your kids melt with these fun ice cream learning resources!
- Ice Cream Letter Puzzle Printables
- Ice Cream Books for Preschoolers
- Ice Cream Activities for First Grade
- Ice Cream Worksheets for Kindergarten
- Splat the Cat Ice Cream Book Companion by Homeschool Preschool
- I is for Ice Cream Handprint Craft by Homeschool of 1
Conducting science experiments with ice cream engages children in hands-on science learning, sparking curiosity and creativity at a young age. These activities also teach basic scientific concepts like freezing points and simple chemical reactions.
Additionally, they foster teamwork and problem-solving skills, all while having fun and enjoying a delicious summer treat after.
These exciting ice cream science experiments will make learning a deliciously fun adventure for kids!

Ice Cream Cone Volcanoes
Find out how baking soda reacts with vinegar in this explosion of fun in chemistry with this ice cream-themed volcano experiment.

How to Make Ice Cream Quicker using Heat Transfer!
Uncover the reason behind this fun phenomenon of making ice cream solid extremely fast using heat!

Ice Cream Melting Exploration
Let your kids explore the outdoors during this summer and take this fun ice cream melting activity as an opportunity for another cool science experiment!

How to Make Chemistry Amazing by Making Ice Cream
Learn chemistry with your kids by creating something edible and to be enjoyed by them after with this fun science experiment.

Tin Can Ice Cream Science Experiment
Create this yummy summer treat with a few ingredients available at home and a fun and unique material - a tin can!

Ice Cream in a Bag: Changing Matter Experiment for Second Grade
Let your kids observe how states matter change from liquid to solid through this fun science experiment involving your child's favorite cold treat!

How to Make Fluffy Ice Cream Slime
Recreate your child's favorite cold dessert using a few simple ingredients in this fun chemistry experiment making fluffy ice cream slime for kids!

Ice Cream in a Bag Science Experiment
Another fun and cool way of making ice cream the simplest way possible with your little food techs!
So what are you waiting for? Grab some simple ingredients and transform your kitchen or your den into a science lab with these delightful ice cream experiments that moms and kids will love doing together!
I share educational printables and activities to help homeschoolers make learning science fun and engaging!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
- Skip to main content
- Skip to primary sidebar
Second Grade teachers! Join me Around the 2nd Grade Kampfire on Facebook! JOIN HERE
- Facebook Group
- Search this website
Around the Kampfire
Elementary Teaching Blog
Last updated by Linda Kamp on December 10, 2022 • 17 Comments
Ice Cream in a Bag: Changing Matter Experiment for Second Grade
In second grade science, students investigate ways that matter can change and whether these changes are reversible. In this post, I’ll show you a fun changing matter experiment you can easily do at school by making ice cream in a bag! It’s a delicious demonstration of how temperature can change matter from a liquid to a solid that students can eat once they finish!

Changing Matter Experiment for Second Grade
Did you know that ice cream is a solid, a liquid, and a gas all at once? It’s true. Ice cream is a combination of solid ice crystals, liquid milk fat, and air bubbles. Making ice cream is a fun and easy way for students to investigate how matter is changed by heating and cooling. As they make their ice cream, students also gain experience in carrying out investigations , making observations, and collecting and analyzing data.
To get started you will need a few inexpensive items that are readily available at any grocery store. I even found a half gallon size jug of half-and-half.

Materials per student:
- 1 cup half-and-half per student
- 2 Tbs. sugar
- 1/2 tsp. vanilla extract
- 1/3 cup rock salt or kosher salt
- sandwich size zipper bag
- 1 gallon size zipper bag per 2 students
- plastic spoons
- student lab sheet
Multiply the recipe by the number of students you have. I recommend making a large batch of this recipe in a pitcher ahead of time.
1. Mix the half-and-half, sugar, and vanilla extract together in a pitcher.
2. Pour about a cup of the mixture in each student’s sandwich bag.
3. Fill a gallon size Ziploc bag about half full of ice. Add 1/3 cup of salt.
4. Place 2 sealed, sandwich bags with the mixture in each large bag. Seal the bag firmly.
5. Students take turns shaking the bag vigorously for about 7-10 minutes, pausing to record changes they observe on their lab sheet.

Source: Changing Matter Experiment
Analyzing the Changing Matter
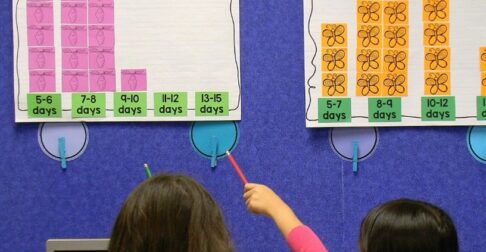
Have students describe the state of their mixture at the start. Next, they record changes they observed after cooling and shaking the mixture for 1 minute, 5 minutes, and 10 minutes. After the investigation, have students compare their data with other students to determine an average time it takes to freeze the mixture. Have students discuss whether they think the amount of shaking is a factor.
As students are completing their lab sheets, encourage them to describe the properties of their ice cream including color, shape (the mixture takes the shape of its container while liquid), and texture.
I recommend making an extra bag of ice cream to demonstrate reversing the change. This can be done by placing the ice cream bag in a sunny window or leaving it out on a counter to melt.

The Science Behind Ice Cream
Adding salt to ice lowers its melting point. The ice absorbs heat from its surroundings (the bag of ice cream mixture) which allows the ice cream mixture to freeze. Since the mixture isn’t water it needs to be a little below 32 degrees to freeze. Adding salt allows the temperature around the mixture to get colder.
Find more properties of matter science activities in this unit: Properties of Matter unit for 2nd grade .

Get Free Science Activities
Try these engaging science lessons at home or in the classroom! This FREE science mini-unit includes both printable + digital activities on Google Slides that make it easy to learn science no matter where you are!
I hope you will try this changing matter experiment and give your students hands-on experience in demonstrating a reversible change. Be sure to pin this post for later so you have it when you plan!

Visit these posts for more hands-on, high engagement science activities:
Marvelous Ways to Teach Matter

Plant Life Cycle Activities

Sharpie Solubility Experiment

Happy teaching!
Share this:

Yearlong assessments
Free Grammar Toolkit
Assess all second grade grammar and language standards with this yearlong assessment tool!

You May Also Enjoy These Posts
Reader Interactions
17 comments.
April 10 at 9:34 am
Hi! Student teacher here. Is there any way I can purchase just this ice cream activity? Thank you in advance! Would love to do this with my second grade class.
March 23 at 6:35 am
Hi! I have a student who is dairy free. Do you know if this would work with dairy-free half and half? Thanks!
March 23 at 7:12 am
Hi Sarah, I haven’t tried making ice cream with dairy free half and half. If you do try it, I’d love to know if it works!
March 8 at 3:27 pm
Is there a printable with this for collecting the data for the ice cream experiment?
January 3 at 11:47 am
Hi. Where can I get a copy of the lab sheet?
November 8 at 7:55 am
Thanks for sharing this interactive and hands-on experiment!
November 13 at 10:08 am
You’re welcome Esther! I hope your students enjoy it!
March 24 at 9:47 am
Is there any way to buy the ice cream recording sheets and not the entire matter unit? We already taught matter but it was when we were virtual. I want to do this experiment after field day next week! (Trying to make this year as normal as possible!)
Leave a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Notify me of new posts by email.
Hello Friends
I’m Linda Kamp, a 20 year primary grade teacher with a passion for creating educational materials that excite students and make learning fun! I'm so glad you're here!


COMMENTS
In your ice cream the fat molecules in the cream are perfectly mixed with water, ice crystals, sugar, and small pockets of air to form a delicious cold treat. ... Chemical technicians conduct experiments, record data, and help to implement new processes and procedures in the laboratory. If you enjoy hands-on work, then you might be interested ...
Dry ice ice cream works a lot like liquid nitrogen ice cream in that the dry ice freezes the recipe. The dry ice undergoes sublimation, changing from a solid directly into carbon dioxide gas (no liquid phase). Because carbon dioxide is more reactive than nitrogen, some dissolves, reacts with the ingredients, and even forms bubbles.
What is ice cream? Ice cream is made up of droplets of fat from milk jumbled up with millions of tiny crystals of ice and pockets of air. This activity uses the freezing power of salt and ice to create ice crystals in milk without a freezer! Homemade Ice Cream in a Bag Science Experiment What you need to make ice cream in a bag. A large bag of ...
Whatever milk you choose, a product with a higher fat content produces a creamier ice cream. You might get ice crystals if you use low-fat or skim milk. For the Ice-Salt Mixture: 3 cups ice; 1/3 cup rock salt or kosher salt (we'll talk more about why we use these specific types of salt later) The quantities of ice and salt are not critical ...
Ice cream is an emulsion. In an emulsion, small droplets of one liquid are dispersed (or spread out) throughout another. When you shake the ice cream, you disperse the ice crystals, fat molecules, and air in the other ingredients. The more you shake, the smaller the ice crystals get and the more air you add. This makes the ice cream creamier!
Ice Cream in a Bag Recipe. Making homemade ice cream in a bag is easy and a good arm workout! This ice cream in a bag science experiment is a fun edible science activity to try at home or in the classroom. It does require some adult supervision and assistance. A good pair of gloves is needed, as this science activity gets very cold.
How to make the Science Scoop homemade ice cream experiment. Supplies you will need. For this experiment, you'll need: Sugar (1 tablespoon) Ice (about 5 cups) Half and half (1/2 cup) Salt (1/2 cup) Vanilla extract (1/4 teaspoon) Sealable sandwich bag (1) Sealable gallon-sized bag (1)
Conducting science experiments with ice cream engages children in hands-on science learning, sparking curiosity and creativity at a young age. These activities also teach basic scientific concepts like freezing points and simple chemical reactions. Additionally, they foster teamwork and problem-solving skills, all while having fun and enjoying ...
Changing matter experiment . The Science Behind Ice Cream. Adding salt to ice lowers its melting point. The ice absorbs heat from its surroundings (the bag of ice cream mixture) which allows the ice cream mixture to freeze. Since the mixture isn't water it needs to be a little below 32 degrees to freeze. Adding salt allows the temperature ...
Have you ever made your own ice cream? It can be a lot of fun, and you end up with a tasty frozen treat! In this activity, you will explore the interesting c...