
Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.

10 Experimental research
Experimental research—often considered to be the ‘gold standard’ in research designs—is one of the most rigorous of all research designs. In this design, one or more independent variables are manipulated by the researcher (as treatments), subjects are randomly assigned to different treatment levels (random assignment), and the results of the treatments on outcomes (dependent variables) are observed. The unique strength of experimental research is its internal validity (causality) due to its ability to link cause and effect through treatment manipulation, while controlling for the spurious effect of extraneous variable.
Experimental research is best suited for explanatory research—rather than for descriptive or exploratory research—where the goal of the study is to examine cause-effect relationships. It also works well for research that involves a relatively limited and well-defined set of independent variables that can either be manipulated or controlled. Experimental research can be conducted in laboratory or field settings. Laboratory experiments , conducted in laboratory (artificial) settings, tend to be high in internal validity, but this comes at the cost of low external validity (generalisability), because the artificial (laboratory) setting in which the study is conducted may not reflect the real world. Field experiments are conducted in field settings such as in a real organisation, and are high in both internal and external validity. But such experiments are relatively rare, because of the difficulties associated with manipulating treatments and controlling for extraneous effects in a field setting.
Experimental research can be grouped into two broad categories: true experimental designs and quasi-experimental designs. Both designs require treatment manipulation, but while true experiments also require random assignment, quasi-experiments do not. Sometimes, we also refer to non-experimental research, which is not really a research design, but an all-inclusive term that includes all types of research that do not employ treatment manipulation or random assignment, such as survey research, observational research, and correlational studies.
Basic concepts
Treatment and control groups. In experimental research, some subjects are administered one or more experimental stimulus called a treatment (the treatment group ) while other subjects are not given such a stimulus (the control group ). The treatment may be considered successful if subjects in the treatment group rate more favourably on outcome variables than control group subjects. Multiple levels of experimental stimulus may be administered, in which case, there may be more than one treatment group. For example, in order to test the effects of a new drug intended to treat a certain medical condition like dementia, if a sample of dementia patients is randomly divided into three groups, with the first group receiving a high dosage of the drug, the second group receiving a low dosage, and the third group receiving a placebo such as a sugar pill (control group), then the first two groups are experimental groups and the third group is a control group. After administering the drug for a period of time, if the condition of the experimental group subjects improved significantly more than the control group subjects, we can say that the drug is effective. We can also compare the conditions of the high and low dosage experimental groups to determine if the high dose is more effective than the low dose.
Treatment manipulation. Treatments are the unique feature of experimental research that sets this design apart from all other research methods. Treatment manipulation helps control for the ‘cause’ in cause-effect relationships. Naturally, the validity of experimental research depends on how well the treatment was manipulated. Treatment manipulation must be checked using pretests and pilot tests prior to the experimental study. Any measurements conducted before the treatment is administered are called pretest measures , while those conducted after the treatment are posttest measures .
Random selection and assignment. Random selection is the process of randomly drawing a sample from a population or a sampling frame. This approach is typically employed in survey research, and ensures that each unit in the population has a positive chance of being selected into the sample. Random assignment, however, is a process of randomly assigning subjects to experimental or control groups. This is a standard practice in true experimental research to ensure that treatment groups are similar (equivalent) to each other and to the control group prior to treatment administration. Random selection is related to sampling, and is therefore more closely related to the external validity (generalisability) of findings. However, random assignment is related to design, and is therefore most related to internal validity. It is possible to have both random selection and random assignment in well-designed experimental research, but quasi-experimental research involves neither random selection nor random assignment.
Threats to internal validity. Although experimental designs are considered more rigorous than other research methods in terms of the internal validity of their inferences (by virtue of their ability to control causes through treatment manipulation), they are not immune to internal validity threats. Some of these threats to internal validity are described below, within the context of a study of the impact of a special remedial math tutoring program for improving the math abilities of high school students.
History threat is the possibility that the observed effects (dependent variables) are caused by extraneous or historical events rather than by the experimental treatment. For instance, students’ post-remedial math score improvement may have been caused by their preparation for a math exam at their school, rather than the remedial math program.
Maturation threat refers to the possibility that observed effects are caused by natural maturation of subjects (e.g., a general improvement in their intellectual ability to understand complex concepts) rather than the experimental treatment.
Testing threat is a threat in pre-post designs where subjects’ posttest responses are conditioned by their pretest responses. For instance, if students remember their answers from the pretest evaluation, they may tend to repeat them in the posttest exam.
Not conducting a pretest can help avoid this threat.
Instrumentation threat , which also occurs in pre-post designs, refers to the possibility that the difference between pretest and posttest scores is not due to the remedial math program, but due to changes in the administered test, such as the posttest having a higher or lower degree of difficulty than the pretest.
Mortality threat refers to the possibility that subjects may be dropping out of the study at differential rates between the treatment and control groups due to a systematic reason, such that the dropouts were mostly students who scored low on the pretest. If the low-performing students drop out, the results of the posttest will be artificially inflated by the preponderance of high-performing students.
Regression threat —also called a regression to the mean—refers to the statistical tendency of a group’s overall performance to regress toward the mean during a posttest rather than in the anticipated direction. For instance, if subjects scored high on a pretest, they will have a tendency to score lower on the posttest (closer to the mean) because their high scores (away from the mean) during the pretest were possibly a statistical aberration. This problem tends to be more prevalent in non-random samples and when the two measures are imperfectly correlated.
Two-group experimental designs
Pretest-posttest control group design . In this design, subjects are randomly assigned to treatment and control groups, subjected to an initial (pretest) measurement of the dependent variables of interest, the treatment group is administered a treatment (representing the independent variable of interest), and the dependent variables measured again (posttest). The notation of this design is shown in Figure 10.1.

Statistical analysis of this design involves a simple analysis of variance (ANOVA) between the treatment and control groups. The pretest-posttest design handles several threats to internal validity, such as maturation, testing, and regression, since these threats can be expected to influence both treatment and control groups in a similar (random) manner. The selection threat is controlled via random assignment. However, additional threats to internal validity may exist. For instance, mortality can be a problem if there are differential dropout rates between the two groups, and the pretest measurement may bias the posttest measurement—especially if the pretest introduces unusual topics or content.
Posttest -only control group design . This design is a simpler version of the pretest-posttest design where pretest measurements are omitted. The design notation is shown in Figure 10.2.

The treatment effect is measured simply as the difference in the posttest scores between the two groups:
The appropriate statistical analysis of this design is also a two-group analysis of variance (ANOVA). The simplicity of this design makes it more attractive than the pretest-posttest design in terms of internal validity. This design controls for maturation, testing, regression, selection, and pretest-posttest interaction, though the mortality threat may continue to exist.
Because the pretest measure is not a measurement of the dependent variable, but rather a covariate, the treatment effect is measured as the difference in the posttest scores between the treatment and control groups as:
Due to the presence of covariates, the right statistical analysis of this design is a two-group analysis of covariance (ANCOVA). This design has all the advantages of posttest-only design, but with internal validity due to the controlling of covariates. Covariance designs can also be extended to pretest-posttest control group design.
Factorial designs
Two-group designs are inadequate if your research requires manipulation of two or more independent variables (treatments). In such cases, you would need four or higher-group designs. Such designs, quite popular in experimental research, are commonly called factorial designs. Each independent variable in this design is called a factor , and each subdivision of a factor is called a level . Factorial designs enable the researcher to examine not only the individual effect of each treatment on the dependent variables (called main effects), but also their joint effect (called interaction effects).
In a factorial design, a main effect is said to exist if the dependent variable shows a significant difference between multiple levels of one factor, at all levels of other factors. No change in the dependent variable across factor levels is the null case (baseline), from which main effects are evaluated. In the above example, you may see a main effect of instructional type, instructional time, or both on learning outcomes. An interaction effect exists when the effect of differences in one factor depends upon the level of a second factor. In our example, if the effect of instructional type on learning outcomes is greater for three hours/week of instructional time than for one and a half hours/week, then we can say that there is an interaction effect between instructional type and instructional time on learning outcomes. Note that the presence of interaction effects dominate and make main effects irrelevant, and it is not meaningful to interpret main effects if interaction effects are significant.
Hybrid experimental designs
Hybrid designs are those that are formed by combining features of more established designs. Three such hybrid designs are randomised bocks design, Solomon four-group design, and switched replications design.
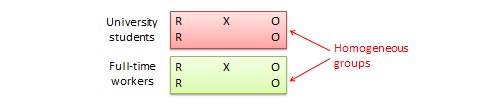
Randomised block design. This is a variation of the posttest-only or pretest-posttest control group design where the subject population can be grouped into relatively homogeneous subgroups (called blocks ) within which the experiment is replicated. For instance, if you want to replicate the same posttest-only design among university students and full-time working professionals (two homogeneous blocks), subjects in both blocks are randomly split between the treatment group (receiving the same treatment) and the control group (see Figure 10.5). The purpose of this design is to reduce the ‘noise’ or variance in data that may be attributable to differences between the blocks so that the actual effect of interest can be detected more accurately.
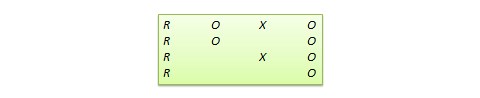
Solomon four-group design . In this design, the sample is divided into two treatment groups and two control groups. One treatment group and one control group receive the pretest, and the other two groups do not. This design represents a combination of posttest-only and pretest-posttest control group design, and is intended to test for the potential biasing effect of pretest measurement on posttest measures that tends to occur in pretest-posttest designs, but not in posttest-only designs. The design notation is shown in Figure 10.6.
Switched replication design . This is a two-group design implemented in two phases with three waves of measurement. The treatment group in the first phase serves as the control group in the second phase, and the control group in the first phase becomes the treatment group in the second phase, as illustrated in Figure 10.7. In other words, the original design is repeated or replicated temporally with treatment/control roles switched between the two groups. By the end of the study, all participants will have received the treatment either during the first or the second phase. This design is most feasible in organisational contexts where organisational programs (e.g., employee training) are implemented in a phased manner or are repeated at regular intervals.
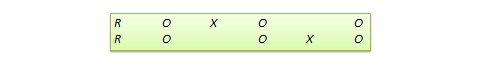
Quasi-experimental designs
Quasi-experimental designs are almost identical to true experimental designs, but lacking one key ingredient: random assignment. For instance, one entire class section or one organisation is used as the treatment group, while another section of the same class or a different organisation in the same industry is used as the control group. This lack of random assignment potentially results in groups that are non-equivalent, such as one group possessing greater mastery of certain content than the other group, say by virtue of having a better teacher in a previous semester, which introduces the possibility of selection bias . Quasi-experimental designs are therefore inferior to true experimental designs in interval validity due to the presence of a variety of selection related threats such as selection-maturation threat (the treatment and control groups maturing at different rates), selection-history threat (the treatment and control groups being differentially impacted by extraneous or historical events), selection-regression threat (the treatment and control groups regressing toward the mean between pretest and posttest at different rates), selection-instrumentation threat (the treatment and control groups responding differently to the measurement), selection-testing (the treatment and control groups responding differently to the pretest), and selection-mortality (the treatment and control groups demonstrating differential dropout rates). Given these selection threats, it is generally preferable to avoid quasi-experimental designs to the greatest extent possible.
In addition, there are quite a few unique non-equivalent designs without corresponding true experimental design cousins. Some of the more useful of these designs are discussed next.
Regression discontinuity (RD) design . This is a non-equivalent pretest-posttest design where subjects are assigned to the treatment or control group based on a cut-off score on a preprogram measure. For instance, patients who are severely ill may be assigned to a treatment group to test the efficacy of a new drug or treatment protocol and those who are mildly ill are assigned to the control group. In another example, students who are lagging behind on standardised test scores may be selected for a remedial curriculum program intended to improve their performance, while those who score high on such tests are not selected from the remedial program.
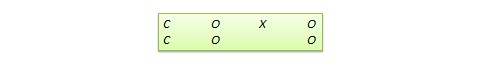
Because of the use of a cut-off score, it is possible that the observed results may be a function of the cut-off score rather than the treatment, which introduces a new threat to internal validity. However, using the cut-off score also ensures that limited or costly resources are distributed to people who need them the most, rather than randomly across a population, while simultaneously allowing a quasi-experimental treatment. The control group scores in the RD design do not serve as a benchmark for comparing treatment group scores, given the systematic non-equivalence between the two groups. Rather, if there is no discontinuity between pretest and posttest scores in the control group, but such a discontinuity persists in the treatment group, then this discontinuity is viewed as evidence of the treatment effect.
Proxy pretest design . This design, shown in Figure 10.11, looks very similar to the standard NEGD (pretest-posttest) design, with one critical difference: the pretest score is collected after the treatment is administered. A typical application of this design is when a researcher is brought in to test the efficacy of a program (e.g., an educational program) after the program has already started and pretest data is not available. Under such circumstances, the best option for the researcher is often to use a different prerecorded measure, such as students’ grade point average before the start of the program, as a proxy for pretest data. A variation of the proxy pretest design is to use subjects’ posttest recollection of pretest data, which may be subject to recall bias, but nevertheless may provide a measure of perceived gain or change in the dependent variable.
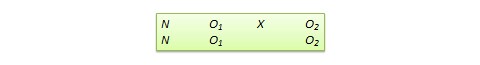
Separate pretest-posttest samples design . This design is useful if it is not possible to collect pretest and posttest data from the same subjects for some reason. As shown in Figure 10.12, there are four groups in this design, but two groups come from a single non-equivalent group, while the other two groups come from a different non-equivalent group. For instance, say you want to test customer satisfaction with a new online service that is implemented in one city but not in another. In this case, customers in the first city serve as the treatment group and those in the second city constitute the control group. If it is not possible to obtain pretest and posttest measures from the same customers, you can measure customer satisfaction at one point in time, implement the new service program, and measure customer satisfaction (with a different set of customers) after the program is implemented. Customer satisfaction is also measured in the control group at the same times as in the treatment group, but without the new program implementation. The design is not particularly strong, because you cannot examine the changes in any specific customer’s satisfaction score before and after the implementation, but you can only examine average customer satisfaction scores. Despite the lower internal validity, this design may still be a useful way of collecting quasi-experimental data when pretest and posttest data is not available from the same subjects.

An interesting variation of the NEDV design is a pattern-matching NEDV design , which employs multiple outcome variables and a theory that explains how much each variable will be affected by the treatment. The researcher can then examine if the theoretical prediction is matched in actual observations. This pattern-matching technique—based on the degree of correspondence between theoretical and observed patterns—is a powerful way of alleviating internal validity concerns in the original NEDV design.
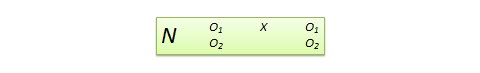
Perils of experimental research
Experimental research is one of the most difficult of research designs, and should not be taken lightly. This type of research is often best with a multitude of methodological problems. First, though experimental research requires theories for framing hypotheses for testing, much of current experimental research is atheoretical. Without theories, the hypotheses being tested tend to be ad hoc, possibly illogical, and meaningless. Second, many of the measurement instruments used in experimental research are not tested for reliability and validity, and are incomparable across studies. Consequently, results generated using such instruments are also incomparable. Third, often experimental research uses inappropriate research designs, such as irrelevant dependent variables, no interaction effects, no experimental controls, and non-equivalent stimulus across treatment groups. Findings from such studies tend to lack internal validity and are highly suspect. Fourth, the treatments (tasks) used in experimental research may be diverse, incomparable, and inconsistent across studies, and sometimes inappropriate for the subject population. For instance, undergraduate student subjects are often asked to pretend that they are marketing managers and asked to perform a complex budget allocation task in which they have no experience or expertise. The use of such inappropriate tasks, introduces new threats to internal validity (i.e., subject’s performance may be an artefact of the content or difficulty of the task setting), generates findings that are non-interpretable and meaningless, and makes integration of findings across studies impossible.
The design of proper experimental treatments is a very important task in experimental design, because the treatment is the raison d’etre of the experimental method, and must never be rushed or neglected. To design an adequate and appropriate task, researchers should use prevalidated tasks if available, conduct treatment manipulation checks to check for the adequacy of such tasks (by debriefing subjects after performing the assigned task), conduct pilot tests (repeatedly, if necessary), and if in doubt, use tasks that are simple and familiar for the respondent sample rather than tasks that are complex or unfamiliar.
In summary, this chapter introduced key concepts in the experimental design research method and introduced a variety of true experimental and quasi-experimental designs. Although these designs vary widely in internal validity, designs with less internal validity should not be overlooked and may sometimes be useful under specific circumstances and empirical contingencies.
Social Science Research: Principles, Methods and Practices (Revised edition) Copyright © 2019 by Anol Bhattacherjee is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book

What is Experimental Research: Definition, Types & Examples
Understand how experimental research enables researchers to confidently identify causal relationships between variables and validate findings, enhancing credibility.
June 16, 2024

In this Article
Short on time? Get an AI generated summary of this article instead
AI-generated article summary
This blog covers the essentials of experimental research and its application in businesses. It emphasizes the value of controlled and systematic research design for establishing cause-and-effect relationships, managing variables, and generating reliable results. The blog explains the types of experimental research, including pre-experimental, true experimental, and quasi-experimental designs, each with distinct structures that fit different research needs. The importance of experimental research is outlined through examples such as product testing, marketing optimization, pricing strategies, and customer experience enhancement. These examples show how businesses can use experimental research to test hypotheses, improve products, and make informed decisions. Despite its benefits, the blog also highlights the disadvantages, like ethical concerns, artificial settings, high costs, and participant biases. Finally, the blog suggests leveraging tools like Entropik's Decode for AI-driven market research, which allows businesses to experiment and analyze consumer behavior for better decision-making. The post concludes with a brief FAQ section, answering common questions about experimental research, including examples and its application in various fields like medicine, psychology, and education.
Get fast AI summaries of customer calls and feedback with magic summarize in Decode
Experimental research is crucial for companies because it allows them to precisely control and measure key factors, identify dependent and independent elements, and set conditions to observe their effects. By changing one variable systematically, it is possible to determine possible cause-and-effect relations and analyze how specific observed effects depend on them.
Read this blog to learn more about how experimental research design can drive business success and provide practical examples of its application in various industries.
What is Experimental Research?
Experimental research is a systematic and scientific approach in which the researcher manipulates one or more independent variables and observes the effect on a dependent variable while controlling for extraneous variables. This method allows for the establishment of cause-and-effect relationships between variables.
Experimental research involves using control groups, random assignment, and standardized procedures to ensure the reliability and validity of the results. It is commonly used in psychology, medicine, and the social sciences to test hypotheses and theories under controlled conditions.
Example of Experimental Research
An experimental research example scenario can be a clinical trial for a new medication. This scenario aims to determine whether the new type of drug applies to the patient. Accordingly, patients with hypertension diagnosed by a medical practitioner are randomly assigned to two groups.
The experimental group is subjected to the new medication the research treatment facility delivers. In contrast, the control group is treated with either a placebo or the medical drugs previously used by the patients. The data will be both quantitative and qualitative .
Quantitative data will include blood pressure levels or symptom severity scores. Qualitative data will include symptoms reported by the patient or symptoms observed by the practitioner and side effects experienced by the patients. Consequently, the research on which type of drug is effective is tested, and results are obtained by comparing the patient's conditions in the two groups.
Researchers believe a new medication works if the experimental group shows significant symptom improvements compared to a control group and has no immediate side effects. Testing many patients increases confidence that the effects are due to the medication and not a placebo effect.
What Are The Different Types of Experimental Research?
The following are the different types of experimental research: Pre-Experimental Research
- One-Shot Case Study: A single group is exposed to a treatment and then observed for outcomes. There is no control group for comparison.
- One-Group Pretest-Posttest Design: A single group is measured before and after treatment to observe changes.
True Experimental Research
- Randomized Controlled Trials (RCT): Participants are randomly assigned to experimental and control groups to ensure comparability and reduce bias. This design is considered the gold standard in experimental research.
- Pretest-Posttest Control Group Design: Both the experimental and control groups are measured before and after the treatment. The experimental group receives the treatment, while the control group does not.
- Posttest-Only Control Group Design: Participants are randomly assigned to experimental and control groups, but measurements are taken only after the treatment is administered to the experimental group.
Quasi-Experimental Research
- Non-Equivalent Groups Design: Similar to the pretest-posttest control group design, participants are not randomly assigned to groups. This design is often used when random assignment is not feasible.
- Interrupted Time Series Design: Multiple measurements are taken before and after a treatment to observe changes over time. This design helps control time-related variables.
- Matched Groups Design: Participants are matched based on certain characteristics before being assigned to experimental and control groups, ensuring comparable groups.
Factorial Design
- Full Factorial Design: Involves manipulating two or more independent variables simultaneously to observe their interaction effects on the dependent variable. All possible combinations of the independent variables are tested.
- Fractional Factorial Design: A subset of the possible combinations of independent variables is tested, making it more practical when dealing with many variables.
What is the Importance of Experimental Research?

Establishing Causality
Experimental research is essential for establishing correlations between variables of interest and demonstrating causality. It allows researchers to manipulate one or more independent variables considered the cause and record changes in the dependent variable, the effect.
Controlling Variables
One of the strengths of this type of research is that it allows for controlling the effect of extraneous variables. This means that experimental research reduces alternative explanations of effects. Using control groups and random assignment to conditions, the experimental method can accurately determine whether the observed group differences resulted from manipulating the independent variable or other factors.
Providing Reliable and Valid Results
A structured and rigorous methodology while conducting experimental research minimizes the possibility of measurement errors and biases. In addition, randomized controlled trials are generally accepted as the gold standard in research. Because of these features, the data’s reliability can be confirmed in advance by similar findings, and the results will also be more replicable and generalizable to the broader population.
Informing Decision-Making
Experimental research provides empirical evidence and data to support important organizational decisions, such as product testing and experimentation, marketing strategies, or improving operational processes and activities.
Driving Innovation
Experimental research drives innovation by systematically testing new ideas and interventions. It allows companies and researchers to experiment with novel concepts in a controlled environment, identify successful innovations, and confidently scale them up.
What Are The Disadvantages of Experimental Research?
Ethical concerns.
Experimental research implies ethical dilemmas, especially when human subjects are concerned. Generally speaking, ethical principles prohibit manipulating variables, specifically intentionally causing harm to, offending, and inducing psychological or physical pressure. Ethics guidelines and review boards are expected to curb risks in an experiment, but they could also somewhat restrain findings.
Artificial Settings
Most experimental studies are conducted in highly controlled artificial conditions, such as laboratories, where external variables are properly controlled and isolated. Thus, the conclusions of the findings might only sometimes be extended to the real world, so they will only sometimes be applicable. The main type of validity under which this problem falls is external validity. Some variables cannot be controlled or do not appear in artificial conditions.
High Costs and Time Consumption
Experimental research is expensive and time-consuming. There are various reasons for this statement. First, such a type of research requires specialized equipment, controlled conditions of measurement, and large sample sizes, which means increased costs. Second, designing an experiment, preparing all the necessary information and tools for its implementation, running it, and analyzing the data received is usually time-consuming, even in the simplest cases.
Practical and Logistical Constraints
Some variables or phenomena cannot be either manipulated or controlled. Experimental studies are impractical if processes are complex, large-scale, or long-term. For example, a lab cannot treat anything related to the environment or societal changes. Therefore, due to the inability to conduct experiments based on such phenomena, some questions can only be studied by other experimental research methods, such as observational or correlational.
Participant Behavior and Bias
Experimental studies may be biased based on the participants’ awareness of being observed during the process. Also called the Hawthorne effect, another issue that can hurt the study’s validity, especially in medical research, is using control groups. Although they are necessary to measure the efficiency of a certain treatment, such research may involve not providing some groups with potentially beneficial treatment.
These two problems may affect the results and make them unethical. In either case, corrective steps should be taken to address this issue and ensure that the results have been obtained properly.
How Businesses Can Leverage Experimental Research?
Product development and testing.
Businesses can use experimental research to test new products or features before launching them. By creating controlled experiments, such as A/B testing , companies can compare different versions of a product to see which one performs better in terms of customer satisfaction, usability, and sales. This approach allows businesses to refine their products based on empirical evidence, reducing the risk of failure upon release.
Marketing Strategy Optimization
Experimental research is invaluable for optimizing marketing strategies. Businesses can test different marketing messages, channels, and tactics to determine which are most effective in engaging their target audience and driving conversions. For example, they can conduct randomized controlled trials to compare the impact of various advertising campaigns on consumer behavior , enabling data-driven decisions that enhance marketing ROI.
Customer Experience Enhancement
Customer experience is increasingly more critical for retention and loyalty. Companies use experimental research to determine the best practices for customer service, website design, and in-store experience. Through experimenting and measuring responses, companies can identify what promotes satisfaction and loyalty and apply these results to enhance customer experience.
Pricing Strategies
Experimental research helps businesses determine optimal pricing strategies. Companies can analyze consumer reactions and willingness to pay by testing different price points in controlled settings. This approach enables businesses to find the price that maximizes revenue without deterring customers, balancing profitability with market competitiveness.
Operational Efficiency
Businesses can use experimental research to enhance operational efficiency. For instance, they can test various processes, workflows, or technologies to identify which ones improve productivity, reduce costs, or enhance quality. Companies can implement the most effective strategies and practices by systematically experimenting with different operational changes, leading to better overall performance.
Final Words
Experimental research has become a powerful instrument for modern business development. It systematically tests assumptions and variables associated with various activities, from product development, marketing strategies, and customer experiences to pricing and operational efficiencies.

Get your hands on Decode , an AI-powered market research tool that can help you test hypotheses about consumer behavior and preferences. Companies can determine cause-and-effect relationships by manipulating specific variables, such as pricing or advertising methods, and observing the effects on consumer responses using Decode diary studies .
This research method collects qualitative data on user behaviors, activities, and experiences over time. This helps them make informed decisions about product development, marketing strategies, and overall business operations.
Frequently Asked Questions (FAQs)
Question 1: what are examples of experimental research.
Answer: Examples of experimental research include drug trials, psychology experiments, and studies testing new teaching methods. These experiments involve manipulating variables and comparing outcomes to establish causal relationships.
Question 2: What is the meaning of experimental design in research?
Answer: Experimental design in research refers to the methodical planning of experiments to control variables, minimize bias, and draw valid conclusions. It involves carefully considering factors like sample size, randomization, and control groups.
Question 3: What are the characteristics of experimental research?
Answer: Characteristics of experimental research include manipulation of variables, random assignment, control groups, and measurement of outcomes. These features ensure that researchers can isolate the effects of specific variables and draw reliable conclusions.
Question 4: Where is experimental research used?
Answer: Experimental research is used in medicine, psychology, education, and natural sciences to investigate cause-and-effect relationships and validate hypotheses. It provides a systematic approach to testing theories and informing evidence-based practices.
Frequently Asked Questions
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Suspendisse varius enim in eros elementum tristique. Duis cursus, mi quis viverra ornare, eros dolor interdum nulla, ut commodo diam libero vitae erat. Aenean faucibus nibh et justo cursus id rutrum lorem imperdiet. Nunc ut sem vitae risus tristique posuere.
With lots of unique blocks, you can easily build a page without coding.
Click on Study templates
Start from scratch
Add blocks to the content
Saving the Template
Publish the Template
Soham is a true Manchester United fan who finds joy in more than just football. Whether navigating the open road, scoring virtual goals in FIFA, reading novels, or enjoying quality time with friends, Soham embraces a life full of diverse passions.
Product Marketing Specialist
Related Articles

Unlocking Consumer Insights: The Power of Conjoint Analysis
Discover consumer preferences with conjoint analysis. Optimize product features, pricing, and decisions for impactful, data-driven insights

Toggle Buttons: A UX Designer’s Secret Weapon
Toggle buttons are powerful UX tools that simplify user interactions by enabling effortless switching between two states, such as on/off or show/hide. They enhance usability with intuitive design, visual clarity, and accessibility, making them ideal for settings menus, filters, and dashboards. Thoughtful design elements like clear labels, strong contrast, and instant feedback ensure toggles are both functional and delightful. This blog explores the versatility of toggles, their impact on user experience, and best practices for designing effective toggle buttons.
.jpg)
Trend Analysis in Market Research: Staying Ahead of the Curve
Discover how trend analysis in market research helps predict shifts, identify opportunities, and drive informed decisions with tools like social listening, predictive analytics, and Emotion AI.

The Ultimate Guide to Crafting Research Plans: Key Steps, Tips, and Tools
A comprehensive guide to crafting effective research plans with key steps, tips, and tools like Decode & Qatalyst for actionable insights and success.

Roadmap to Building an Effective Research Question
Learn the essentials of crafting impactful research questions. Discover strategies to frame and refine questions for clearer, more actionable insights.
Synthetic Users: Revolutionizing UX Testing and Digital Performance
Synthetic users simulate real behavior to enhance UX testing and digital performance, enabling businesses to optimize experiences and detect issues pre-emptively.
Correlation vs Causation: Applied in UX Research
Discover the critical distinctions between correlation and causation and why they matter in UX Research.

Subjective vs Objective Research: A Competitive Analysis
Explore the differences between subjective and objective research methods, their impact on data analysis, and how to apply each approach effectively.

Predictive Analytics: Harnessing the Power to Foresee the Future
AI-Driven Predictive Analytics: The Key to Forecasting Market Trends, Boosting Efficiency, and Accelerating Business Growth

Mastering the Art of Conceptual Framework in Market Research
Learn how a conceptual framework in market research guides your study, connects theory with practice and ensures a clear structure for effective analysis and results.

The Ultimate User Testing Guide
Explore our guide on user testing, covering its importance, methods, and how to enhance user experience through actionable insights. Read more!

Stratified Random Sampling: A Complete Guide with Definition, Method, and Examples
Master Stratified Random Sampling: A Step-by-Step Guide to Boost Precision in Research and Decision-Making for Researchers and Product Managers

Building Customer Loyalty: Best Practices and Strategies
Customer loyalty is a cornerstone of any successful business. This comprehensive guide will explore various strategies and tactics to help you cultivate a loyal customer base.

How to Create Buyer Personas That Drive Product and Marketing Success?
Develop detailed, data-backed buyer personas to improve product development, refine marketing strategies, and deliver personalized experiences.

What is a Survey? Benefits, Types, Blocks, Use Cases, and More
Discover the power of surveys: types, benefits, use cases, and how to create effective surveys using the AI-powered platform Decode.
Top AI Events You Do Not Want to Miss in 2024
Here are all the top AI events for 2024, curated in one convenient place just for you.

Top Insights Events You Do Not Want to Miss in 2024
Here are all the top Insights events for 2024, curated in one convenient place just for you.

Top CX Events You Do Not Want to Miss in 2024
Here are all the top CX events for 2024, curated in one convenient place just for you.

How to Build an Experience Map: A Complete Guide
An experience map is essential for businesses, as it highlights the customer journey, uncovering insights to improve user experiences and address pain points. Read to find more!

Everything You Need to Know about Intelligent Scoring
Are you curious about Intelligent Scoring and how it differs from regular scoring? Discover its applications and benefits. Read on to learn more!

Qualitative Research Methods and Its Advantages In Modern User Research
Discover how to leverage qualitative research methods, including moderated sessions, to gain deep user insights and enhance your product and UX decisions.

The 10 Best Customer Experience Platforms to Transform Your CX
Explore the top 10 CX platforms to revolutionize customer interactions, enhance satisfaction, and drive business growth.

TAM SAM SOM: What It Means and How to Calculate It?
Understanding TAM, SAM, SOM helps businesses gauge market potential. Learn their definitions and how to calculate them for better business decisions and strategy.

Understanding Likert Scales: Advantages, Limitations, and Questions
Using Likert scales can help you understand how your customers view and rate your product. Here's how you can use them to get the feedback you need.

Mastering the 80/20 Rule to Transform User Research
Find out how the Pareto Principle can optimize your user research processes and lead to more impactful results with the help of AI.
Understanding Consumer Psychology: The Science Behind What Makes Us Buy
Gain a comprehensive understanding of consumer psychology and learn how to apply these insights to inform your research and strategies.

A Guide to Website Footers: Best Design Practices & Examples
Explore the importance of website footers, design best practices, and how to optimize them using UX research for enhanced user engagement and navigation.

Customer Effort Score: Definition, Examples, Tips
A great customer score can lead to dedicated, engaged customers who can end up being loyal advocates of your brand. Here's what you need to know about it.

How to Detect and Address User Pain Points for Better Engagement
Understanding user pain points can help you provide a seamless user experiences that makes your users come back for more. Here's what you need to know about it.

What is Quota Sampling? Definition, Types, Examples, and How to Use It?
Discover Quota Sampling: Learn its process, types, and benefits for accurate consumer insights and informed marketing decisions. Perfect for researchers and brand marketers!

What Is Accessibility Testing? A Comprehensive Guide
Ensure inclusivity and compliance with accessibility standards through thorough testing. Improve user experience and mitigate legal risks. Learn more.

Maximizing Your Research Efficiency with AI Transcriptions
Explore how AI transcription can transform your market research by delivering precise and rapid insights from audio and video recordings.

Understanding the False Consensus Effect: How to Manage it
The false consensus effect can cause incorrect assumptions and ultimately, the wrong conclusions. Here's how you can overcome it.

5 Banking Customer Experience Trends to Watch Out for in 2024
Discover the top 5 banking customer experience trends to watch out for in 2024. Stay ahead in the evolving financial landscape.

The Ultimate Guide to Regression Analysis: Definition, Types, Usage & Advantages
Master Regression Analysis: Learn types, uses & benefits in consumer research for precise insights & strategic decisions.
EyeQuant Alternative
Meet Qatalyst, your best eyequant alternative to improve user experience and an AI-powered solution for all your user research needs.
EyeSee Alternative
Embrace the Insights AI revolution: Meet Decode, your innovative solution for consumer insights, offering a compelling alternative to EyeSee.

Skeuomorphism in UX Design: Is It Dead?
Skeuomorphism in UX design creates intuitive interfaces using familiar real-world visuals to help users easily understand digital products. Do you know how?

Top 6 Wireframe Tools and Ways to Test Your Designs
Wireframe tools assist designers in planning and visualizing the layout of their websites. Look through this list of wireframing tools to find the one that suits you best.

Revolutionizing Customer Interaction: The Power of Conversational AI
Conversational AI enhances customer service across various industries, offering intelligent, context-aware interactions that drive efficiency and satisfaction. Here's how.

User Story Mapping: A Powerful Tool for User-Centered Product Development
Learn about user story mapping and how it can be used for successful product development with this blog.

What is Research Hypothesis: Definition, Types, and How to Develop
Read the blog to learn how a research hypothesis provides a clear and focused direction for a study and helps formulate research questions.

Understanding Customer Retention: How to Keep Your Customers Coming Back
Understanding customer retention is key to building a successful brand that has repeat, loyal customers. Here's what you need to know about it.

Demographic Segmentation: How Brands Can Use it to Improve Marketing Strategies
Read this blog to learn what demographic segmentation means, its importance, and how it can be used by brands.

Mastering Product Positioning: A UX Researcher's Guide
Read this blog to understand why brands should have a well-defined product positioning and how it affects the overall business.

Discrete Vs. Continuous Data: Everything You Need To Know
Explore the differences between discrete and continuous data and their impact on business decisions and customer insights.

50+ Employee Engagement Survey Questions
Understand how an employee engagement survey provides insights into employee satisfaction and motivation, directly impacting productivity and retention.

A Guide to Interaction Design
Interaction design can help you create engaging and intuitive user experiences, improving usability and satisfaction through effective design principles. Here's how.

Exploring the Benefits of Stratified Sampling
Understanding stratified sampling can improve research accuracy by ensuring diverse representation across key subgroups. Here's how.

A Guide to Voice Recognition in Enhancing UX Research
Learn the importance of using voice recognition technology in user research for enhanced user feedback and insights.


The Ultimate Figma Design Handbook: Design Creation and Testing
The Ultimate Figma Design Handbook covers setting up Figma, creating designs, advanced features, prototyping, and testing designs with real users.
The Power of Organization: Mastering Information Architectures
Understanding the art of information architectures can enhance user experiences by organizing and structuring digital content effectively, making information easy to find and navigate. Here's how.
Convenience Sampling: Examples, Benefits, and When To Use It
Read the blog to understand how convenience sampling allows for quick and easy data collection with minimal cost and effort.
What is Critical Thinking, and How Can it be Used in Consumer Research?
Learn how critical thinking enhances consumer research and discover how Decode's AI-driven platform revolutionizes data analysis and insights.

How Business Intelligence Tools Transform User Research & Product Management
This blog explains how Business Intelligence (BI) tools can transform user research and product management by providing data-driven insights for better decision-making.

What is Face Validity? Definition, Guide and Examples
Read this blog to explore face validity, its importance, and the advantages of using it in market research.

What is Customer Lifetime Value, and How To Calculate It?
Read this blog to understand how Customer Lifetime Value (CLV) can help your business optimize marketing efforts, improve customer retention, and increase profitability.

Systematic Sampling: Definition, Examples, and Types
Explore how systematic sampling helps researchers by providing a structured method to select representative samples from larger populations, ensuring efficiency and reducing bias.

Understanding Selection Bias: A Guide
Selection bias can affect the type of respondents you choose for the study and ultimately the quality of responses you receive. Here’s all you need to know about it.

A Guide to Designing an Effective Product Strategy
Read this blog to explore why a well-defined product strategy is required for brands while developing or refining a product.

A Guide to Minimum Viable Product (MVP) in UX: Definition, Strategies, and Examples
Discover what an MVP is, why it's crucial in UX, strategies for creating one, and real-world examples from top companies like Dropbox and Airbnb.

Asking Close Ended Questions: A Guide
Asking the right close ended questions is they key to getting quantitiative data from your users. Her's how you should do it.

Creating Website Mockups: Your Ultimate Guide to Effective Design
Read this blog to learn website mockups- tools, examples and how to create an impactful website design.

Understanding Your Target Market And Its Importance In Consumer Research
Read this blog to learn about the importance of creating products and services to suit the needs of your target audience.

What Is a Go-To-Market Strategy And How to Create One?
Check out this blog to learn how a go-to-market strategy helps businesses enter markets smoothly, attract more customers, and stand out from competitors.
What is Confirmation Bias in Consumer Research?
Learn how confirmation bias affects consumer research, its types, impacts, and practical tips to avoid it for more accurate and reliable insights.

Market Penetration: The Key to Business Success
Understanding market penetration is key to cracking the code to sustained business growth and competitive advantage in any industry. Here's all you need to know about it.

How to Create an Effective User Interface
Having a simple, clear user interface helps your users find what they really want, improving the user experience. Here's how you can achieve it.
Product Differentiation and What It Means for Your Business
Discover how product differentiation helps businesses stand out with unique features, innovative designs, and exceptional customer experiences.

What is Ethnographic Research? Definition, Types & Examples
Read this blog to understand Ethnographic research, its relevance in today’s business landscape and how you can leverage it for your business.

Product Roadmap: The 2024 Guide [with Examples]
Read this blog to understand how a product roadmap can align stakeholders by providing a clear product development and delivery plan.

Product Market Fit: Making Your Products Stand Out in a Crowded Market
Delve into the concept of product-market fit, explore its significance, and equip yourself with practical insights to achieve it effectively.
Consumer Behavior in Online Shopping: A Comprehensive Guide
Ever wondered how online shopping behavior can influence successful business decisions? Read on to learn more.

How to Conduct a First Click Test?
Why are users leaving your site so fast? Learn how First Click Testing can help. Discover quick fixes for frustration and boost engagement.

What is Market Intelligence? Methods, Types, and Examples
Read the blog to understand how marketing intelligence helps you understand consumer behavior and market trends to inform strategic decision-making.
What is a Longitudinal Study? Definition, Types, and Examples
Is your long-term research strategy unclear? Learn how longitudinal studies decode complexity. Read on for insights.

What Is the Impact of Customer Churn on Your Business?
Understanding and reducing customer churn is the key to building a healthy business that keeps customers satisfied. Here's all you need to know about it.

The Ultimate Design Thinking Guide
Discover the power of design thinking in UX design for your business. Learn the process and key principles in our comprehensive guide.

100+ Yes Or No Survey Questions Examples
Yes or no survey questions simplify responses, aiding efficiency, clarity, standardization, quantifiability, and binary decision-making. Read some examples!

What is Customer Segmentation? The ULTIMATE Guide
Explore how customer segmentation targets diverse consumer groups by tailoring products, marketing, and experiences to their preferred needs.
Crafting User-Centric Websites Through Responsive Web Design
Find yourself reaching for your phone instead of a laptop for regular web browsing? Read on to find out what that means & how you can leverage it for business.

How Does Product Placement Work? Examples and Benefits
Read the blog to understand how product placement helps advertisers seek subtle and integrated ways to promote their products within entertainment content.

The Importance of Reputation Management, and How it Can Make or Break Your Brand
A good reputation management strategy is crucial for any brand that wants to keep its customers loyal. Here's how brands can focus on it.

A Comprehensive Guide to Human-Centered Design
Are you putting the human element at the center of your design process? Read this blog to understand why brands must do so.

How to Leverage Customer Insights to Grow Your Business
Genuine insights are becoming increasingly difficult to collect. Read on to understand the challenges and what the future holds for customer insights.

The Complete Guide to Behavioral Segmentation
Struggling to reach your target audience effectively? Discover how behavioral segmentation can transform your marketing approach. Read more in our blog!

Creating a Unique Brand Identity: How to Make Your Brand Stand Out
Creating a great brand identity goes beyond creating a memorable logo - it's all about creating a consistent and unique brand experience for your cosnumers. Here's everything you need to know about building one.

Understanding the Product Life Cycle: A Comprehensive Guide
Understanding the product life cycle, or the stages a product goes through from its launch to its sunset can help you understand how to market it at every stage to create the most optimal marketing strategies.

Empathy vs. Sympathy in UX Research
Are you conducting UX research and seeking guidance on conducting user interviews with empathy or sympathy? Keep reading to discover the best approach.

What is Exploratory Research, and How To Conduct It?
Read this blog to understand how exploratory research can help you uncover new insights, patterns, and hypotheses in a subject area.

First Impressions & Why They Matter in User Research
Ever wonder if first impressions matter in user research? The answer might surprise you. Read on to learn more!

Cluster Sampling: Definition, Types & Examples
Read this blog to understand how cluster sampling tackles the challenge of efficiently collecting data from large, spread-out populations.

Top Six Market Research Trends
Curious about where market research is headed? Read on to learn about the changes surrounding this field in 2024 and beyond.
Lyssna Alternative
Meet Qatalyst, your best lyssna alternative to usability testing, to create a solution for all your user research needs.
What is Feedback Loop? Definition, Importance, Types, and Best Practices
Struggling to connect with your customers? Read the blog to learn how feedback loops can solve your problem!

UI vs. UX Design: What’s The Difference?
Learn how UI solves the problem of creating an intuitive and visually appealing interface and how UX addresses broader issues related to user satisfaction and overall experience with the product or service.

The Impact of Conversion Rate Optimization on Your Business
Understanding conversion rate optimization can help you boost your online business. Read more to learn all about it.

Insurance Questionnaire: Tips, Questions and Significance
Leverage this pre-built customizable questionnaire template for insurance to get deep insights from your audience.

UX Research Plan Template
Read on to understand why you need a UX Research Plan and how you can use a fully customizable template to get deep insights from your users!
Maximize Your Research Potential
Experience why teams worldwide trust our Consumer & User Research solutions.
Book a Demo

experimental
Search nci's dictionary of cancer terms.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

1.10: Chapter 10 Experimental Research
- Last updated
- Save as PDF
- Page ID 84786

- William Pelz
- Herkimer College via Lumen Learning
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Experimental research, often considered to be the “gold standard” in research designs, is one of the most rigorous of all research designs. In this design, one or more independent variables are manipulated by the researcher (as treatments), subjects are randomly assigned to different treatment levels (random assignment), and the results of the treatments on outcomes (dependent variables) are observed. The unique strength of experimental research is its internal validity (causality) due to its ability to link cause and effect through treatment manipulation, while controlling for the spurious effect of extraneous variable.
Experimental research is best suited for explanatory research (rather than for descriptive or exploratory research), where the goal of the study is to examine cause-effect relationships. It also works well for research that involves a relatively limited and well-defined set of independent variables that can either be manipulated or controlled. Experimental research can be conducted in laboratory or field settings. Laboratory experiments , conducted in laboratory (artificial) settings, tend to be high in internal validity, but this comes at the cost of low external validity (generalizability), because the artificial (laboratory) setting in which the study is conducted may not reflect the real world. Field experiments , conducted in field settings such as in a real organization, and high in both internal and external validity. But such experiments are relatively rare, because of the difficulties associated with manipulating treatments and controlling for extraneous effects in a field setting.
Experimental research can be grouped into two broad categories: true experimental designs and quasi-experimental designs. Both designs require treatment manipulation, but while true experiments also require random assignment, quasi-experiments do not. Sometimes, we also refer to non-experimental research, which is not really a research design, but an all-inclusive term that includes all types of research that do not employ treatment manipulation or random assignment, such as survey research, observational research, and correlational studies.
Basic Concepts
Treatment and control groups. In experimental research, some subjects are administered one or more experimental stimulus called a treatment (the treatment group ) while other subjects are not given such a stimulus (the control group ). The treatment may be considered successful if subjects in the treatment group rate more favorably on outcome variables than control group subjects. Multiple levels of experimental stimulus may be administered, in which case, there may be more than one treatment group. For example, in order to test the effects of a new drug intended to treat a certain medical condition like dementia, if a sample of dementia patients is randomly divided into three groups, with the first group receiving a high dosage of the drug, the second group receiving a low dosage, and the third group receives a placebo such as a sugar pill (control group), then the first two groups are experimental groups and the third group is a control group. After administering the drug for a period of time, if the condition of the experimental group subjects improved significantly more than the control group subjects, we can say that the drug is effective. We can also compare the conditions of the high and low dosage experimental groups to determine if the high dose is more effective than the low dose.
Treatment manipulation. Treatments are the unique feature of experimental research that sets this design apart from all other research methods. Treatment manipulation helps control for the “cause” in cause-effect relationships. Naturally, the validity of experimental research depends on how well the treatment was manipulated. Treatment manipulation must be checked using pretests and pilot tests prior to the experimental study. Any measurements conducted before the treatment is administered are called pretest measures , while those conducted after the treatment are posttest measures.
Random selection and assignment. Random selection is the process of randomly drawing a sample from a population or a sampling frame. This approach is typically employed in survey research, and assures that each unit in the population has a positive chance of being selected into the sample. Random assignment is however a process of randomly assigning subjects to experimental or control groups. This is a standard practice in true experimental research to ensure that treatment groups are similar (equivalent) to each other and to the control group, prior to treatment administration. Random selection is related to sampling, and is therefore, more closely related to the external validity (generalizability) of findings. However, random assignment is related to design, and is therefore most related to internal validity. It is possible to have both random selection and random assignment in well-designed experimental research, but quasi-experimental research involves neither random selection nor random assignment.
Threats to internal validity. Although experimental designs are considered more rigorous than other research methods in terms of the internal validity of their inferences (by virtue of their ability to control causes through treatment manipulation), they are not immune to internal validity threats. Some of these threats to internal validity are described below, within the context of a study of the impact of a special remedial math tutoring program for improving the math abilities of high school students.
- History threat is the possibility that the observed effects (dependent variables) are caused by extraneous or historical events rather than by the experimental treatment. For instance, students’ post-remedial math score improvement may have been caused by their preparation for a math exam at their school, rather than the remedial math program.
- Maturation threat refers to the possibility that observed effects are caused by natural maturation of subjects (e.g., a general improvement in their intellectual ability to understand complex concepts) rather than the experimental treatment.
- Testing threat is a threat in pre-post designs where subjects’ posttest responses are conditioned by their pretest responses. For instance, if students remember their answers from the pretest evaluation, they may tend to repeat them in the posttest exam. Not conducting a pretest can help avoid this threat.
- Instrumentation threat , which also occurs in pre-post designs, refers to the possibility that the difference between pretest and posttest scores is not due to the remedial math program, but due to changes in the administered test, such as the posttest having a higher or lower degree of difficulty than the pretest.
- Mortality threat refers to the possibility that subjects may be dropping out of the study at differential rates between the treatment and control groups due to a systematic reason, such that the dropouts were mostly students who scored low on the pretest. If the low-performing students drop out, the results of the posttest will be artificially inflated by the preponderance of high-performing students.
- Regression threat , also called a regression to the mean, refers to the statistical tendency of a group’s overall performance on a measure during a posttest to regress toward the mean of that measure rather than in the anticipated direction. For instance, if subjects scored high on a pretest, they will have a tendency to score lower on the posttest (closer to the mean) because their high scores (away from the mean) during the pretest was possibly a statistical aberration. This problem tends to be more prevalent in non-random samples and when the two measures are imperfectly correlated.
Two-Group Experimental Designs
The simplest true experimental designs are two group designs involving one treatment group and one control group, and are ideally suited for testing the effects of a single independent variable that can be manipulated as a treatment. The two basic two-group designs are the pretest-posttest control group design and the posttest-only control group design, while variations may include covariance designs. These designs are often depicted using a standardized design notation, where R represents random assignment of subjects to groups, X represents the treatment administered to the treatment group, and O represents pretest or posttest observations of the dependent variable (with different subscripts to distinguish between pretest and posttest observations of treatment and control groups).
Pretest-posttest control group design. In this design, subjects are randomly assigned to treatment and control groups, subjected to an initial (pretest) measurement of the dependent variables of interest, the treatment group is administered a treatment (representing the independent variable of interest), and the dependent variables measured again (posttest). The notation of this design is shown in Figure 10.1.

Up to 65% off on all yearly plans! 🎁 Start fresh with a yearly plan. Now 65% off! ❄️ 🏷️
- Form Builder
- Survey Maker
- AI Form Generator
- AI Survey Tool
- AI Quiz Maker
- Store Builder
- WordPress Plugin
HubSpot CRM
Google Sheets
Google Analytics
Microsoft Excel
- Popular Forms
- Job Application Form Template
- Rental Application Form Template
- Hotel Accommodation Form Template
- Online Registration Form Template
- Employment Application Form Template
- Application Forms
- Booking Forms
- Consent Forms
- Contact Forms
- Donation Forms
- Customer Satisfaction Surveys
- Employee Satisfaction Surveys
- Evaluation Surveys
- Feedback Surveys
- Market Research Surveys
- Personality Quiz Template
- Geography Quiz Template
- Math Quiz Template
- Science Quiz Template
- Vocabulary Quiz Template
Try without registration Quick Start
Read engaging stories, how-to guides, learn about forms.app features.
Inspirational ready-to-use templates for getting started fast and powerful.
Spot-on guides on how to use forms.app and make the most out of it.
See the technical measures we take and learn how we keep your data safe and secure.
- Integrations
- Help Center
- Sign In Sign Up Free
- What is experimental research: Definition, types & examples
Defne Çobanoğlu
Life and its secrets can only be proven right or wrong with experimentation. You can speculate and theorize all you wish, but as William Blake once said, “ The true method of knowledge is experiment. ”
It may be a long process and time-consuming, but it is rewarding like no other. And there are multiple ways and methods of experimentation that can help shed light on matters. In this article, we explained the definition, types of experimental research, and some experimental research examples . Let us get started with the definition!
- What is experimental research?
Experimental research is the process of carrying out a study conducted with a scientific approach using two or more variables. In other words, it is when you gather two or more variables and compare and test them in controlled environments.
With experimental research, researchers can also collect detailed information about the participants by doing pre-tests and post-tests to learn even more information about the process. With the result of this type of study, the researcher can make conscious decisions.
The more control the researcher has over the internal and extraneous variables, the better it is for the results. There may be different circumstances when a balanced experiment is not possible to conduct. That is why are are different research designs to accommodate the needs of researchers.
- 3 Types of experimental research designs
There is more than one dividing point in experimental research designs that differentiates them from one another. These differences are about whether or not there are pre-tests or post-tests done and how the participants are divided into groups. These differences decide which experimental research design is used.
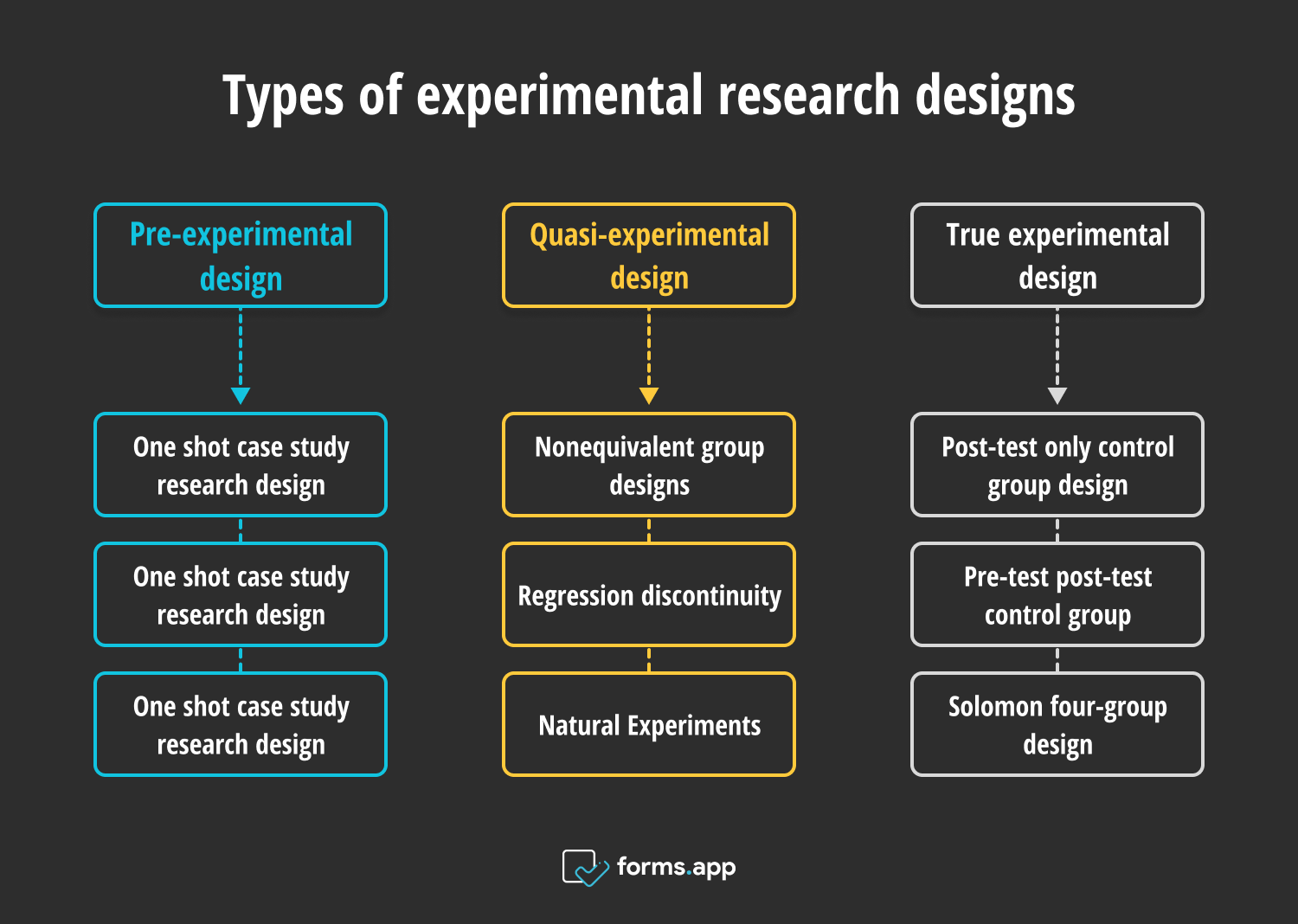
Types of experimental research designs
1 - Pre-experimental design
This is the most basic method of experimental study. The researcher doing pre-experimental research evaluates a group of dependent variables after changing the independent variables . The results of this scientific method are not satisfactory, and future studies are planned accordingly. The pre-experimental research can be divided into three types:
A. One shot case study research design
Only one variable is considered in this one-shot case study design. This research method is conducted in the post-test part of a study, and the aim is to observe the changes in the effect of the independent variable.
B. One group pre-test post-test research design
In this type of research, a single group is given a pre-test before a study is conducted and a post-test after the study is conducted. The aim of this one-group pre-test post-test research design is to combine and compare the data collected during these tests.
C. Static-group comparison
In a static group comparison, 2 or more groups are included in a study where only a group of participants is subjected to a new treatment and the other group of participants is held static. After the study is done, both groups do a post-test evaluation, and the changes are seen as results.
2 - Quasi-experimental design
This research type is quite similar to the experimental design; however, it changes in a few aspects. Quasi-experimental research is done when experimentation is needed for accurate data, but it is not possible to do one because of some limitations. Because you can not deliberately deprive someone of medical treatment or give someone harm, some experiments are ethically impossible. In this experimentation method, the researcher can only manipulate some variables. There are three types of quasi-experimental design:
A. Nonequivalent group designs
A nonequivalent group design is used when participants can not be divided equally and randomly for ethical reasons. Because of this, different variables will be more than one, unlike true experimental research.
B. Regression discontinuity
In this type of research design, the researcher does not divide a group into two to make a study, instead, they make use of a natural threshold or pre-existing dividing point. Only participants below or above the threshold get the treatment, and as the divide is minimal, the difference would be minimal as well.
C. Natural Experiments
In natural experiments, random or irregular assignment of patients makes up control and study groups. And they exist in natural scenarios. Because of this reason, they do not qualify as true experiments as they are based on observation.
3 - True experimental design
In true experimental research, the variables, groups, and settings should be identical to the textbook definition. Grouping of the participant are divided randomly, and controlled variables are chosen carefully. Every aspect of a true experiment should be carefully designed and acted out. And only the results of a true experiment can really be fully accurate . A true experimental design can be divided into 3 parts:
A. Post-test only control group design
In this experimental design, the participants are divided into two groups randomly. They are called experimental and control groups. Only the experimental group gets the treatment, while the other one does not. After the experiment and observation, both groups are given a post-test, and a conclusion is drawn from the results.
B. Pre-test post-test control group
In this method, the participants are divided into two groups once again. Also, only the experimental group gets the treatment. And this time, they are given both pre-tests and post-tests with multiple research methods. Thanks to these multiple tests, the researchers can make sure the changes in the experimental group are directly related to the treatment.
C. Solomon four-group design
This is the most comprehensive method of experimentation. The participants are randomly divided into 4 groups. These four groups include all possible permutations by including both control and non-control groups and post-test or pre-test and post-test control groups. This method enhances the quality of the data.
- Advantages and disadvantages of experimental research
Just as with any other study, experimental research also has its positive and negative sides. It is up to the researchers to be mindful of these facts before starting their studies. Let us see some advantages and disadvantages of experimental research:
Advantages of experimental research:
- All the variables are in the researchers’ control, and that means the researcher can influence the experiment according to the research question’s requirements.
- As you can easily control the variables in the experiment, you can specify the results as much as possible.
- The results of the study identify a cause-and-effect relation .
- The results can be as specific as the researcher wants.
- The result of an experimental design opens the doors for future related studies.
Disadvantages of experimental research:
- Completing an experiment may take years and even decades, so the results will not be as immediate as some of the other research types.
- As it involves many steps, participants, and researchers, it may be too expensive for some groups.
- The possibility of researchers making mistakes and having a bias is high. It is important to stay impartial
- Human behavior and responses can be difficult to measure unless it is specifically experimental research in psychology.
- Examples of experimental research
When one does experimental research, that experiment can be about anything. As the variables and environments can be controlled by the researcher, it is possible to have experiments about pretty much any subject. It is especially crucial that it gives critical insight into the cause-and-effect relationships of various elements. Now let us see some important examples of experimental research:
An example of experimental research in science:
When scientists make new medicines or come up with a new type of treatment, they have to test those thoroughly to make sure the results will be unanimous and effective for every individual. In order to make sure of this, they can test the medicine on different people or creatures in different dosages and in different frequencies. They can double-check all the results and have crystal clear results.
An example of experimental research in marketing:
The ideal goal of a marketing product, advertisement, or campaign is to attract attention and create positive emotions in the target audience. Marketers can focus on different elements in different campaigns, change the packaging/outline, and have a different approach. Only then can they be sure about the effectiveness of their approaches. Some methods they can work with are A/B testing, online surveys , or focus groups .
- Frequently asked questions about experimental research
Is experimental research qualitative or quantitative?
Experimental research can be both qualitative and quantitative according to the nature of the study. Experimental research is quantitative when it provides numerical and provable data. The experiment is qualitative when it provides researchers with participants' experiences, attitudes, or the context in which the experiment is conducted.
What is the difference between quasi-experimental research and experimental research?
In true experimental research, the participants are divided into groups randomly and evenly so as to have an equal distinction. However, in quasi-experimental research, the participants can not be divided equally for ethical or practical reasons. They are chosen non-randomly or by using a pre-existing threshold.
- Wrapping it up
The experimentation process can be long and time-consuming but highly rewarding as it provides valuable as well as both qualitative and quantitative data. It is a valuable part of research methods and gives insight into the subjects to let people make conscious decisions.
In this article, we have gathered experimental research definition, experimental research types, examples, and pros & cons to work as a guide for your next study. You can also make a successful experiment using pre-test and post-test methods and analyze the findings. For further information on different research types and for all your research information, do not forget to visit our other articles!
Defne is a content writer at forms.app. She is also a translator specializing in literary translation. Defne loves reading, writing, and translating professionally and as a hobby. Her expertise lies in survey research, research methodologies, content writing, and translation.
- Form Features
- Data Collection
Table of Contents
Related posts.

What are the 4Ps of marketing: Definition, examples & how to use
Işılay Kırbaş
.png)
15 Best SurveySparrow alternatives in 2024 (features & prices)

The best form builder list for 2022
forms.app Team
study guides for every class
That actually explain what's on your next test, experimental studies, from class:, intro to epidemiology.
Experimental studies are research designs in which researchers actively manipulate one or more variables to observe the effects on an outcome, allowing for the establishment of cause-and-effect relationships. These studies often involve controlled trials, such as randomized controlled trials (RCTs), where participants are assigned to different groups to receive or not receive an intervention. The design of experimental studies is crucial in understanding the effectiveness of public health interventions and contributes significantly to the evolution of epidemiology and its evidence base.
congrats on reading the definition of Experimental Studies . now let's actually learn it.
5 Must Know Facts For Your Next Test
- Experimental studies are considered the gold standard for establishing causal relationships in epidemiology due to their ability to control for confounding variables.
- These studies typically involve a hypothesis that researchers test through systematic investigation and manipulation of independent variables.
- Randomization in experimental studies helps reduce bias by ensuring that each participant has an equal chance of being assigned to any group, enhancing the validity of results.
- Blinding can be used in experimental studies to prevent bias, where participants and/or researchers are unaware of group assignments.
- Ethical considerations are paramount in experimental studies, especially when involving human subjects, requiring informed consent and careful oversight.
Review Questions
- Experimental studies are essential in establishing causal relationships because they involve the manipulation of variables and control over conditions. By randomizing participants into different groups and applying specific interventions, researchers can isolate the effects of those interventions on outcomes. This design minimizes confounding factors that might skew results, leading to more reliable conclusions about causality in public health interventions.
- When conducting experimental studies with human subjects, researchers must prioritize ethical considerations such as obtaining informed consent, ensuring confidentiality, and minimizing harm. Participants should fully understand the study's purpose, procedures, risks, and benefits before agreeing to participate. Additionally, oversight by institutional review boards (IRBs) ensures that research meets ethical standards, protecting participants from potential exploitation or adverse effects.
- The evolution of experimental studies has significantly strengthened epidemiologic evidence by providing a robust framework for testing hypotheses about interventions' effectiveness. These studies allow for rigorous evaluation through controlled conditions and randomization, leading to high-quality evidence that can inform public health policy and practice. However, limitations still exist, such as challenges in generalizability to broader populations and ethical constraints on manipulating certain exposures. Overall, while experimental studies enhance evidence quality, their contextual relevance must be carefully assessed.
Related terms
Randomized Controlled Trial (RCT) : A type of experimental study where participants are randomly assigned to either the intervention group or the control group to assess the effectiveness of an intervention.
Intervention : An action or treatment implemented to modify a health outcome, often assessed in experimental studies to evaluate its efficacy.
A group of participants in an experimental study that does not receive the intervention, serving as a benchmark to measure the effects of the intervention on the experimental group.
" Experimental Studies " also found in:
Subjects ( 15 ).
- AP Statistics
- Adult Nursing Care
- Advanced Communication Research Methods
- Business Cognitive Bias
- Improvisational Leadership
- Intro to Epistemology
- Intro to Political Research
- Intro to Sociolinguistics
- Language and Cognition
- Media Effects
- Microbiology
- Psychology of Economic Decision-Making
- Psychology of Language
- Public Health Policy and Administration
- Social Psychology
© 2024 Fiveable Inc. All rights reserved.
Ap® and sat® are trademarks registered by the college board, which is not affiliated with, and does not endorse this website..

IMAGES
COMMENTS
Random assignment, however, is a process of randomly assigning subjects to experimental or control groups. This is a standard practice in true experimental research to ensure that treatment groups are similar (equivalent) to each other and to the control group prior to treatment administration.
What is Experimental Research? Experimental research is a systematic and scientific approach in which the researcher manipulates one or more independent variables and observes the effect on a dependent variable while controlling for extraneous variables. This method allows for the establishment of cause-and-effect relationships between variables.
Overall, true experimental designs are sometimes difficult to implement in a real-world practice environment. It may be impossible to withhold treatment from a control group or randomly assign participants in a study. In these cases, pre-experimental and quasi-experimental designs–which we will discuss in the next section–can be used ...
In clinical trials, refers to a drug (including a new drug, dose, combination, or route of administration) or procedure that has undergone basic laboratory testing and received approval from the U.S. Food and Drug Administration (FDA) to be tested in human subjects.
This is a standard practice in true experimental research to ensure that treatment groups are similar (equivalent) to each other and to the control group, prior to treatment administration. Random selection is related to sampling, and is therefore, more closely related to the external validity (generalizability) of findings.
Mar 1, 2016 · Experimental public administration can be strengthened by increasing diversification in terms of samples, experimental designs, experimental types and substantive scope.
Nov 6, 2023 · A true experimental design can be divided into 3 parts: A. Post-test only control group design. In this experimental design, the participants are divided into two groups randomly. They are called experimental and control groups. Only the experimental group gets the treatment, while the other one does not.
Administration scholars that can improve the quality of their experiments. Keywords: experimental method in the behavioral sciences; research methods in public administration; reproducibility. O método experimental na Administração Pública: algumas lições das replicações na Psicologia
the condition of an experiment that contrasts with the experimental condition and serves as a comparison for evaluating the effect of the treatment random assignment assigning participants to experimental and control conditions by chance, thus minimizing preexisting differences between those assigned to the different groups
Experimental studies are research designs in which researchers actively manipulate one or more variables to observe the effects on an outcome, allowing for the establishment of cause-and-effect relationships. These studies often involve controlled trials, such as randomized controlled trials (RCTs), where participants are assigned to different groups to receive or not receive an intervention ...