FESTIVAL PERKS WITH OUTSIDE+
Don’t miss Khruangbin, Lord Huron, and more at the Outside Festival.
GET TICKETS NOW
MUSIC ANNOUNCED!
Khruangbin and Lord Huron to headline 2025 Outside Festival.
BUY TICKETS
If you buy through our links, we may earn an affiliate commission. This supports our mission to get more people active and outside. Learn more


How Does Your Brain Respond When You Hold Your Breath?
When you stop breathing, oxygen flowing to your brain actually increases—at least for a while

- Share on Facebook
- Share on Reddit
New perk: Easily find new routes and hidden gems, upcoming running events, and more near you. Your weekly Local Running Newsletter has everything you need to lace up! >","name":"in-content-cta","type":"link"}}'>Subscribe today .
We all know what it feels like to run out of oxygen—or at least, what it feels like to feel like we’re running out of oxygen. In reality, the breathlessness we experience during hard exercise, or at high altitude, or when simply holding our breath, has more to do with too much carbon dioxide in the blood than with too little oxygen. As the feats of elite freedivers show—like holding a single breath for 11 minutes and 35 seconds —our limits aren’t what they seem.
I’ve long been fascinated by studies of what’s going on inside freedivers when they hold their breath , what defines their limits, and how those skills may translate to other environments like high altitude . But their abilities are so outlandish that it feels like studying another species. So I was particularly interested to see a recent study in the European Journal of Applied Physiology that looked at breath holding in regular people with no prior training in it. The study is very straightforward, taking measurements of heart rate and oxygen levels while volunteers hold their breath, and it offers a revealing picture of how the body copes with a shortage of oxygen—and what can go wrong.
The research was performed at Ghent University in Belgium, by Janne Bouten, Jan Bourgois, and Jan Boone. (I’m assuming scientists in Belgium are assigned to different departments by alphabetical order.) They asked 31 volunteers (17 men, 14, women) to hold their breath for as long as possible three times in a row, with two minutes break each time. Typically people get better and better in repeated breath holds, in part because their spleens are squeezing more oxygen-carrying red blood cells into circulation. During the third and final breath hold, they took continuous measurements of parameters including heart rate, oxygen levels in the brain, and oxygen levels in the leg muscles.
Humans, like other mammals, have a “diving response” that kicks in when you hold your breath, with the goal of making sure your brain always has enough oxygen. As the researchers point out, if your circulation stops abruptly, you’ll be unconscious within 30 seconds and suffer irreversible damage within two to ten minutes. The diving response is enhanced if your face is submerged in water, but it happens even on dry land. Your heart rate drops, and the blood vessels leading to non-essential parts of the body like your leg muscles constrict in order to redirect crucial blood (and oxygen) to the brain.
The subjects held their third breath for an average of two minutes and 37 seconds, which strikes me as incredibly good for normal untrained people. Maybe doing three breaths in a row is the secret; or maybe I’m just weak. Anyway, here’s what the average heart rate response looked like. The data is only shown for the first 60 seconds (on the left) and the last 60 seconds (on the right), which allows them to plot everyone’s data together even though they lasted differing amounts of time. The gray area indicates when they started and stopped the breath hold.
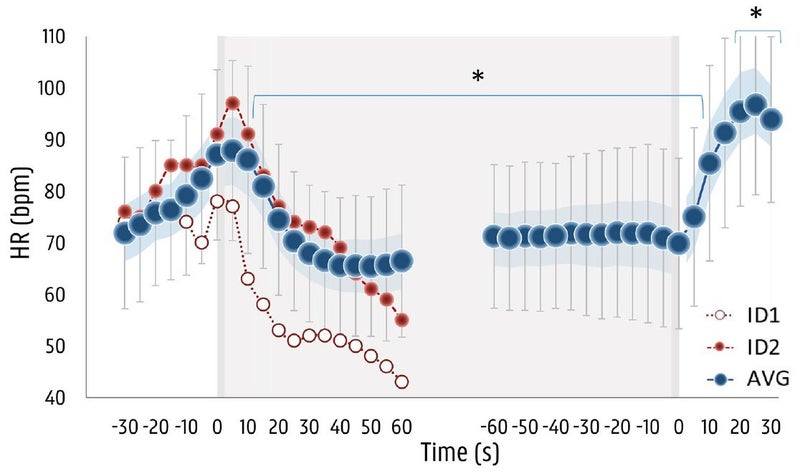
On the far left, you can see the blue dots (which represent the average value) increasing as the subjects prepare for the breath hold. This may be because they’re getting excited or apprehensive, and may also be the result of taking some deep breaths in preparation. The subjects were specifically forbidden from hyperventilating before the breath hold (which blows off a bunch of carbon dioxide, allowing you to hold your breath for longer), but they were given a 30-second warning and a 10-second countdown, and told to take a deep but not maximal breath right before starting. Within about ten seconds after starting the breath hold, heart rate is dropping. It ends up decreasing by 27 beats per minute, reaching its low point after 83 seconds on average. This is fairly similar to what you see in elite free divers, except they reach their minimum heart rate within 30 to 60 seconds.
You’ll notice a series of red dots, and another series of white dots. There are two individuals who quit early; one of them fainted, and the other got dizzy and was on the verge of fainting. More on them below.
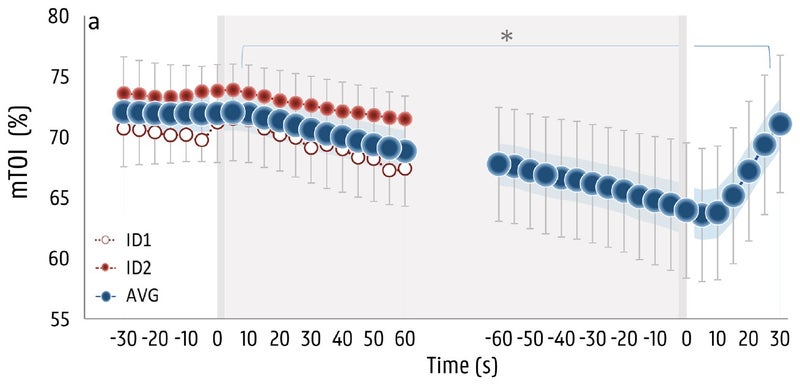
The next parameter is tissue oxygenation in the leg muscles, as measured with near-infrared spectroscopy , which basically involves shining infrared light through the skin and measuring how much is absorbed by oxygen-rich hemoglobin. Here the picture is pretty straightforward: oxygen levels in the muscles start dropping within five seconds, and keep dropping until the subjects start breathing again. This is what you’d expect, because the blood vessels are constricting to shift blood flow away from the extremities to the brain.
The final piece of the puzzle is where things get interesting. Brain oxygenation was also measured with near-infrared spectroscopy:
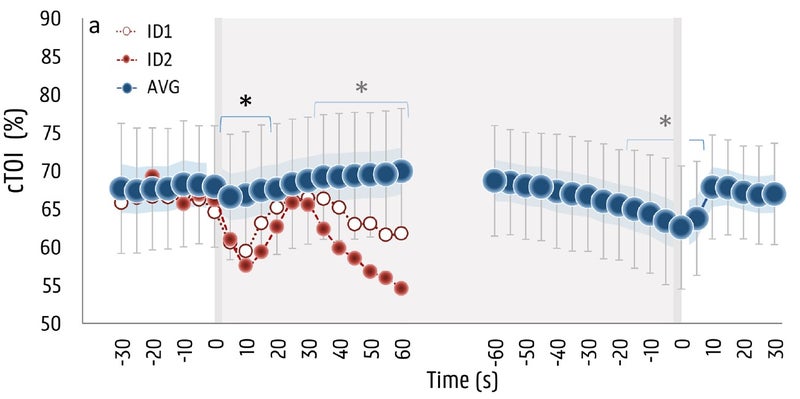
Here you see an initial decrease in brain oxygen levels, perhaps related to the sudden drop in blood pressure associated with the start of a breath hold. But within about five seconds, the drop reverses and brain oxygen levels start to climb—and in fact go on to reach levels about four percent higher than baseline after about a minute. This is a pretty good indication of how powerful the brain’s self-protective wiring is: you hold your breath, and it gets more oxygen rather than less.
That happy state of affairs doesn’t last forever, though. Even as more and more blood gets shunted to the brain, that blood is carrying less and less oxygen as the breath hold proceeds, so gradually your levels of brain oxygen begin to decline. That decline continues until, eventually, you give up. On average, brain oxygen dropped by about five percent by the time the subjects gave up. Interestingly, that’s about the same level you see in elite freedivers after two and a half minutes. That means the freedivers aren’t significantly better at maintaining their brain’s oxygen levels. Instead, the difference seems to be that they’re willing to keep enduring the unpleasant urge to breathe for longer. Other research has found that freedivers are capable of holding their breath until their brain oxygen levels drop so low that they lose consciousness—a very dangerous situation if it happens underwater.
Which brings us back to the two subjects who fainted or came close to it. If you look again at the graph of brain oxygen levels, you can see that their data is way out of whack compared to everyone else’s. They have a steep drop, then manage to compensate for a little while, but the drop resumes and very soon their brain oxygen levels are so low that they reach the border of consciousness. For the red dots, the muscle oxygen data suggests that this subject had a weak response in constricting blood flow to the muscles. That means he or she kept pumping blood to the extremities and didn’t get enough to the brain. For the white dots, the data doesn’t give any hints about what went wrong, but the result was the same: not enough oxygen to the brain.
One of the rationales for the study was that some researchers and coaches have advocated various forms of breath-hold training to improve athletic or altitude performance. Since most previous breath-hold research used trained freedivers, it wasn’t clear whether the brain’s self-protection mechanisms would kick in for novices. The new data indicates that it’s okay, but the two fainters also show that caution is needed: the researchers suggest that everyone should be familiar with the warning signs of fainting (most notably dizziness), and not perform breath-hold training alone.
For more Sweat Science, join me on Twitter and Facebook , sign up for the email newsletter , and check out my book Endure: Mind, Body, and the Curiously Elastic Limits of Human Performance .
- Endurance Training
Popular on Outside Online
Enjoy coverage of racing, history, food, culture, travel, and tech with access to unlimited digital content from Outside Network's iconic brands.
© 2024 Outside Interactive, Inc
Effect of repetitive end-inspiration breath holding on very short-term heart rate variability in healthy humans
Affiliation.
- 1 Key Laboratory of Biomedical Information Engineering of Ministry of Education, Institute of Biomedical Engineering, School of Life Science and Technology, Xi'an Jiaotong University, Xi'an, Shaanxi, People's Republic of China. Postdoctoral Mobile Station of Electronic Engineering, Xi'an Jiaotong University, Xi'an, Shaanxi, People's Republic of China. Solid State Lighting Engineering Research Center, Xi'an Jiaotong University, Xi'an, Shaanxi, People's Republic of China.
- PMID: 25389629
- DOI: 10.1088/0967-3334/35/12/2429
Very short-term heart rate variability (HRV) is thought to reflect dynamic changes in autonomic nervous activity, which is helpful in understanding the role of autonomic nervous function (ANF) in the mechanisms underlying apnea-induced cardiac arrhythmias. The goal of this study was to investigate the effect of repetitive end-inspiration breath holding on very short-term HRV. A total of 32 young healthy participants took part in the experiments. Three trials were performed, each involving seven repetitive end-inspiration breath holding and a 30 s recovery period between breath holding. Durations of breath holding in the three trials were 1:2:3. The study first evaluated the effect of analyzed data lengths on the stability of HRV indices and determined three HRV indices suitable for very short-term analysis. The results showed that in most cases, during breath holding, the square root of the mean squared differences of successive normal RR intervals (rMSSD) was significantly lower, but normalized units of the power in the low frequency band ranging from 0.04 to 0.15 Hz (nLF) and LF/high frequency (HF) were significantly higher than those during corresponding durations under the normal breathing conditions. On the contrary, during recovery after breath holding, rMSSD was significantly higher but nLF and LF/HF were lower than normal. Moreover, the durations of breath holding had no significant influence on the variations of LF/HF. In addition, as participants repeated the breath holding, HRV indices varied non-linearly. HRV changes may indicate sympathetic activation during breath holding and parasympathetic activation during recovery after breath holding. In conjunction with the existing physiological interpretation based on changes in heart rate, the results may imply that breath holding leads to both cardiac sympathetic and parasympathetic activation simultaneously, which may be a possible pathogenic factor of apnea-induced arrhythmias.
Publication types
- Research Support, Non-U.S. Gov't
- Autonomic Nervous System / physiology
- Breath Holding*
- Healthy Volunteers*
- Heart Rate*
- Inhalation / physiology*
- Time Factors
- Young Adult
Advertisement
The interaction of breath holding and muscle mechanoreflex on cardiovascular responses in breath-hold divers and non-breath-hold divers
- Original Article
- Open access
- Published: 05 March 2024
- Volume 124 , pages 2183–2192, ( 2024 )
Cite this article
You have full access to this open access article

- Nakamura Nobuhiro 1 ,
- Peng Heng 2 &
- Hayashi Naoyuki ORCID: orcid.org/0000-0002-9814-3530 1
1409 Accesses
Explore all metrics
Cardiovascular responses to diving are characterized by two opposing responses: tachycardia resulting from exercise and bradycardia resulting from the apnea. The convergence of bradycardia and tachycardia may determine the cardiovascular responses to diving. The purpose of this study was to investigate the interaction of breath holding and muscle mechanoreflex on cardiovascular responses in breath-hold divers (BHDs) and non-BHDs. We compared the cardiovascular responses to combined apnea and the mechanoreflex in BHDs and non-BHDs. All participants undertook three trials—apnea, passive leg cycling (PLC), and combined trials—for 30 s after rest. Cardiovascular variables were measured continuously. Nine BHD (male:female, 4:5; [means ± SD] age, 35 ± 6 years; height, 168.6 ± 4.6 cm; body mass, 58.4 ± 5.9 kg) and eight non-BHD (male:female, 4:4; [means ± SD] age, 35 ± 7 years; height, 163.9 ± 9.1 cm; body mass, 55.6 ± 7.2 kg) participants were included. Compared to the resting baseline, heart rate (HR) and cardiac output (CO) significantly decreased during the combined trial in the BHD group, while they significantly increased during the combined trials in the non-BHD group ( P < 0.05). Changes in the HR and CO were significantly lower in the BHD group than in the non-BHD group in the combined trial ( P < 0.05). These results suggest that bradycardia with apnea in BHDs is prioritized over tachycardia with the mechanoreflex, whereas that in non-BHDs is not. This finding implies that diving training changes the interaction between apnea and the mechanoreflex in cardiovascular control.
Similar content being viewed by others
Heart Rate Variability from Underwater Spiroergometry: How Meaningful?
First Real-Life Data on the Diving Response in Healthy Children
Acute responses of breathing techniques in maximal inspiratory pressure.
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular responses to breath holding are characterized by bradycardia and peripheral vasoconstriction (Lemaître et al. 2008 ). This response plays an important role in preserving the oxygen supply to vital organs such as the heart and brain (Nishiyasu et al. 2012 ; Vestergaard and Larsson 2019 ) and cardiovascular responses induced by the breath-holding reflex may extend the maximal apnea time (Ferretti 2001 ). Breath-hold divers (BHDs) have a greater bradycardia with apnea and longer maximal apnea time than healthy age-matched non-breath-hold divers (non-BHDs) (Joulia et al. 2009 ; Peng et al. 2022 ). The greater bradycardic response to apnea in BHDs may be related to the divers swimming deeper and/or for longer without breathing.
Divers simultaneously perform exercise and apnea during competitive free diving. Cardiovascular responses to diving are characterized by two opposing responses: tachycardia resulting from the physical activity and bradycardia resulting from the apnea. The magnitude of these opposing responses in freediving differ between BHDs and non-BHDs (Hoffmann et al. 2005 ; Tocco et al. 2012 ; Fico et al. 2022 ). Previous studies have suggested that bradycardia resulting from the apnea was predominant over cardiovascular responses resulting from dynamic apnea, that is, freediving, in BHDs (Tocco et al. 2012 ), whereas tachycardia resulting from exercise was predominant in non-BHDs (Hoffmann et al. 2005 ; Fico et al. 2022 ). The interaction of the apnea and exercise on the cardiovascular response and the differences in the interaction between BHDs and non-BHDs require elucidation.
Cardiovascular responses to exercise are influenced by the exercise pressor reflex (EPR), a feedback mechanism resulting from receptors in exercising skeletal muscles (Gallagher et al. 2006 ). The EPR is induced by two stimuli: the mechanoreflex originating from physical changes of exercising muscles (Nóbrega et al. 1994 ; Gladwell and Coote 2002 ; Tokizawa et al. 2004b ; Drew et al. 2008 , 2017 ; Kruse et al. 2016 ; Lis et al. 2020 ; Nakamura et al. 2022 ) and the metaboreflex originating from chemical changes in muscles (Scherrer et al. 1990 ; Tokizawa et al. 2004a ; Ichinose et al. 2006 , 2008 ; Crisafulli et al. 2015 ).
No study has investigated the interaction of the mechanoreflex and apnea, though the effect of the metaboreflex on the apnea has been investigated in both BHDs (Di Giacomo et al. 2021 ) and non-BHDs (Ichinose et al. 2018 ). The metaboreflex mainly affects peripheral vascular tone during dynamic apnea (Ichinose et al. 2018 ; Di Giacomo et al. 2021 ). In turn, the extent of convergence of bradycardia associated with apnea and tachycardia associated with the mechanoreflex is responsible for the cardiac response, as the mechanoreflex in humans primarily contributes to the cardioacceleration during exercise. Thus, the present study aimed to compare the cardiovascular responses in BHDs and non-BHDs to investigate the interaction of the effects of apnea and mechanoreflex on cardiovascular responses. Passive leg cycling (PLC) was used to induce the mechanoreflex (Nóbrega et al. 1994 ; Lis et al. 2020 ).
Materials and mthods
Participants.
We recruited nine BHD (male:female, 4:5; age, 35 ± 6 yr; height, 168.6 ± 4.6 cm; body mass, 58.4 ± 5.9 kg; body mass index [BMI], 20.5 ± 1.2 kg/m 2 ; relative body fat, 19.2 ± 5.1; means ± standard deviation [SD]) and eight non-divers (male:female, 4:4; age, 35 ± 7 yr; height, 163.9 ± 9.1 cm; body mass, 55.6 ± 7.2 kg; BMI, 20.6 ± 1.0 kg/m 2 ; relative body fat, 24.3 ± 6.3%; means ± SD) in this study. The BHDs had personal best of static apnea ≥ 300 s and dynamic with fins ≥ 100 m or constant weight ≥ 40 m. Non-BHDs had not performed habitual aquatic activities and exercise. None of all participants had hypertension (≤ 140/90 mmHg), were smokers, or had cardiovascular disease or diabetes, as assessed by medical history. All participants received verbal and written explanations of the objectives, measurement techniques, and risks and benefits associated with the study and then provided written informed consent to participate before the start of this study. The purpose, procedures, and risks involved in this study were reviewed and approved by the Human Research Committee of Waseda University (approval No. 2021–286). This study was conducted in accordance with the guidelines of the Declaration of Helsinki (1975).
Participants attended the measurement having abstained from strenuous exercise and alcohol for at least 24 h, caffeine for at least 10 h, and food for at least 3 h.
All participants were equipped with measuring devices and rested in a semi-recumbent position for at least 15 min before beginning the protocol (Fig. 1 a). The participants undertook the following three trials (Fig. 1 b); apnea, passive leg cycling (PLC), and combined trials. The order of the trials was randomized to avoid order effects. Trials were separated by a 3 min rest period. One of the investigators was responsible for the randomization to define the order of three trials. The randomization list was created by function of random number generation using spreadsheet software (Microsoft Excel, Microsoft, WA, USA). The randomized crossover trial was performed in open label.
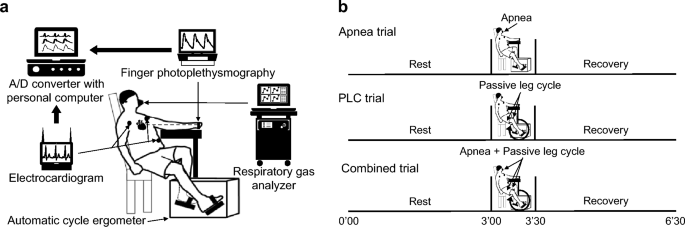
Schematic diagram of the experimental setup ( a ) and overview of the experimental protocol ( b ). Dotted line between heart and finger in panel ( a ) indicates that finger photoplethysmography is located at heart level. PLC passive leg cycling
In the apnea trial, participants performed voluntary apnea with functional residual capacity (FRC) for 30 s. In the PLC trial, passive leg cycling was performed at 60 rpm using automatic cycle ergometer in the semi-recumbent position (AFB3022, Alinco, Osaka, Japan) for 30 s as the mechanoreflex stimulation. Before the PLC trial, the investigator instructed participants to relax to prevent voluntary leg cycling and minimize the unknown effects of other factors such as central command during PLC. No respiratory control was performed during PLC. In the combined trial, participants simultaneously performed apnea and PLC for 30 s. We confirmed breath-holding in the apnea and combined trials using a respiratory gas analyzer (AE-310S, Minato Medical Science Co, Osaka, Japan) with breath-by-breath method. We explained to all participants that FRC is the volume remaining in the lungs after a normal, passive exhalation before the start of the three trials. All participants were instructed to exhale slowly and hold their breath when they reached FRC in apnea and combined trials. All participants familiarized voluntary apnea with FRC and passive leg cycling before beginning the 15 min rest. All measurements were conducted under comfortable laboratory conditions between 14:00 and 18:00.
In an additional experiment on two participants (one BHD and one non-BHD), surface electromyography (EMG) of and vastus lateralis muscles during PLC was recorded to confirm voluntary movement during passive cycle. Signals were amplified by a bioelectric amplifier (MEG-2100, Nihon Kohden, Tokyo, Japan) equipped with input box (JB-210 J, Nihon Kohden, Tokyo, Japan) and obtained at a sampling rate of 1000 Hz through an analog/digital (A/D) converter (PowerLab/16SP, AD Instruments, New South Wales, Australia) and recorded in a device connected to a personal computer (Macbook Pro, Apple, CA, USA).
Body composition
Body composition was measured using bioelectrical impedance analysis (InBody 720; InBody Japan Inc., Tokyo, Japan) with the participant in the upright position.
Cardiovascular variables
The HR and beat-to-beat arterial blood pressure waveforms were monitored using a three-lead electrocardiogram (ECG) (BSM-2401, Nihon Kohden, Tokyo, Japan) and finger photoplethysmography (Finometer MIDI, Finapres Medical Systems, Amsterdam, The Netherlands), respectively. The probe of the latter was attached to the middle finger of the left hand which was located at heart level (Fig. 1 b). Stroke volume (SV) was calculated based on the obtained arterial blood pressure waveform using the model flow method (Wesseling et al. 1993 ), which incorporates age, height, and body mass, and simulates aortic flow waveforms from an arterial pressure signal using a non-linear three-element model of the aortic input impedance (Beatscope, version 1.1, Finapres Medical Systems, Amsterdam, The Netherlands). CO and total peripheral resistance (TPR) were then calculated as SV × HR and mean arterial pressure (MAP) / CO, respectively. All hemodynamic measurements at rest were determined by averaging the values during the last 15 s before the start of each trial. All hemodynamic data were also averaged per 5 s during each trial. The recovery data for 60 s were also determined by averaging the values during the last 15 s.
Heart rate variability
We calculated the HR variability (HRV) to evaluate the cardiac parasympathetic activity. Previous study has reported that the cardiac parasympathetic activity was reflected by the root mean square of standard deviation of R-R intervals (RMSSD) from short-term variation of HR (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996 ). The ECG waveform was obtained at a sampling rate of 1000 Hz through an A/D converter (PowerLab/16SP, AD Instruments, New South Wales, Australia) and recorded in a device connected to a personal computer (Macbook Pro, Apple, CA, USA). Then, the RMSSD were calculated using an analysis software (LabChart8 & MLS 370, AD Instruments, New South Wales, Australia). The RMSSD analysis was conducted by using the last 30 s before the start of trials for the value at rest and the whole 30 s of the trial period.
Statistical analysis
All data are expressed as the mean ± SD. Statistical analyses were performed using IBM SPSS Statistics for Windows version 27.0 (IBM Corp., Armonk, NY, USA). The effects of time and groups were examined using a two-way repeated-measures ANOVA (time × groups). Significant F values were analyzed using Bonferroni’s post hoc test. The changes (Δ) in the HR and CO were calculated as the difference between the 30 s into the trial and the baseline. The mean differences in the characteristics of the participants and the ΔHR and ΔCO between the two groups were examined using the Student’s unpaired t test. The sum of the changes in the apnea and PLC trials was calculated to provide an estimated combined response to compare with the actual response in the combined trial: estimated versus actual responses of convergence of the two inputs from apnea and mechanoreflex. In all of the analyses, the level of significance for all comparisons was set at P < 0.05.
One participant in the BHD group was excluded from this analysis because their ABP waveform was not successfully measured. The results were obtained from the remaining eight participants in the BHD group.
EMG of vastus lateralis muscles showed no obvious activities in the muscle (Fig. 2 ).
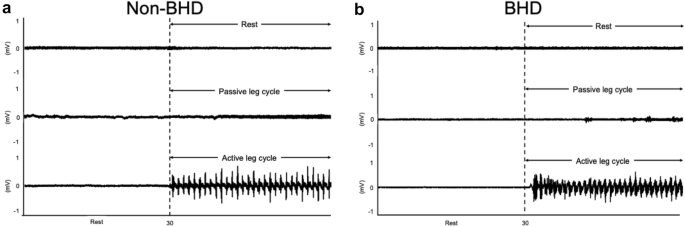
Electromyography recordings of vastus lateralis muscle during passive leg cycling in two subjects; non-BHD ( a ) and BHD ( b ). BHD ;= breath-holding divers
Characteristics of the participants
Table 1 presents the characteristics of the participants. No significant difference was observed in all characteristics of the participants.
Cardiovascular responses
Figure 3 shows the time course of all cardiovascular responses to the apnea and PLC trials compared between the two groups. Two-way analysis of variance revealed a significant interaction between time and group on HR, CO, and TPR in the apnea trial ( P < 0.05; Fig. 3 b, c, and d). The HR and CO during the apnea trial in the BHD group decreased significantly at 25 and 30 s ( P < 0.05). The TPR during the apnea trial in both groups increased significantly at 25 and 30 s ( P < 0.05). The HR during the apnea trial was significantly lower in the BHD group than in the non-BHD group at 25 and 30 s ( P < 0.05). In the PLC trial, the HR and CO in both groups increased significantly at 25 and 30 s ( P < 0.05; Fig. 3 f and g), while the TPR decreased significantly at 25 and 30 s ( P < 0.05; Fig. 3 h).
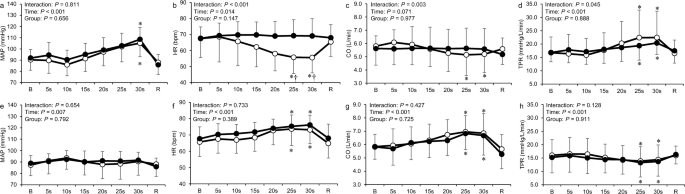
Time course of mean arterial pressure, cardiac output, heart rate, and total peripheral resistance during the apnea ( a – d ) and passive leg cycling ( e – h ) trials in the non-BHD (●) and BHD (○) groups. B baseline; R recovery; CO cardiac output; HR heart rate; MAP mean arterial pressure; TPR total peripheral resistance. Data are presented as mean ± standard deviation. *Significantly different ( P < 0.05) compared with baseline. † Significantly different ( P < 0.05) compared with non-BHD group
Figure 4 shows the cardiovascular responses to the combined trials in the two groups. Two-way ANOVA revealed significant interactions between time and group for HR and CO ( P < 0.05; Fig. 4 b and c). A significant reduction in HR during the combined trial was observed at 20 to 30 s compared with that at baseline in the BHD group, whereas an increase in HR in the non-BHD group at the same time point ( P < 0.05); this indicated a significantly different response between the groups ( P < 0.05). The CO during the combined trial decreased significantly at 25 and 30 s ( P < 0.05), whereas an increase in CO in the non-BHD group at the same time point ( P < 0.05). The CO during the combined trial was significantly lower in the BHD group than in the non-BHD group at 25 and 30 s ( P < 0.05). The MAP and TPR during the combined trial in both groups increased significantly at 25 and 30 s ( P < 0.05; Fig. 4 a and d).
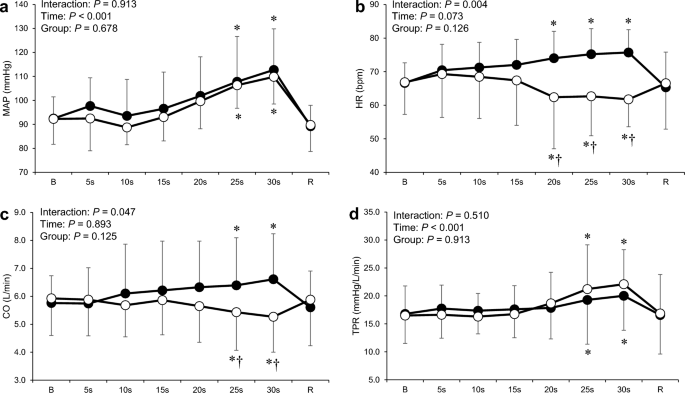
Time course of mean arterial pressure ( a ), heart rate ( b ), cardiac output ( c ), and total peripheral resistance ( d ) during the combined trial in the non-BHD (●) and BHD (○) groups. B baseline; R recovery; CO cardiac output; HR heart rate; MAP mean arterial pressure; TPR total peripheral resistance. Data are presented as mean ± standard deviation. *Significantly different ( P < 0.05) compared with baseline. † Significantly different ( P < 0.05) compared with non-BHD group

Changes in RMSSD
Table 2 shows the RMSSD for all trials. No significant differences were observed between the two groups at baseline. Two-way ANOVA revealed a significant interaction between time and group on RMSSD during the apnea and combined trials ( P < 0.05). A significant increase in the RMSSD was observed during the apnea and combined trials in the BHD group. The RMSSD during the apnea and combined trials were greater in the BHD group than non-BHD group ( P < 0.05). A significant reduction in the RMSSD was also observed during the PLC trial ( P < 0.05).
Changes in heart rate and cardiac output
The ΔHR and ΔCO responses are shown in Fig. 5 . The ΔHR and ΔCO were significantly lower in the BHD group than in the non-BHD group in the apnea and combined trials ( P < 0.05). The estimated responses tended to be positive. In the combined trial, ΔCO was significantly greater in the estimated response than in the actual response ( P < 0.05; Fig. 5 b).
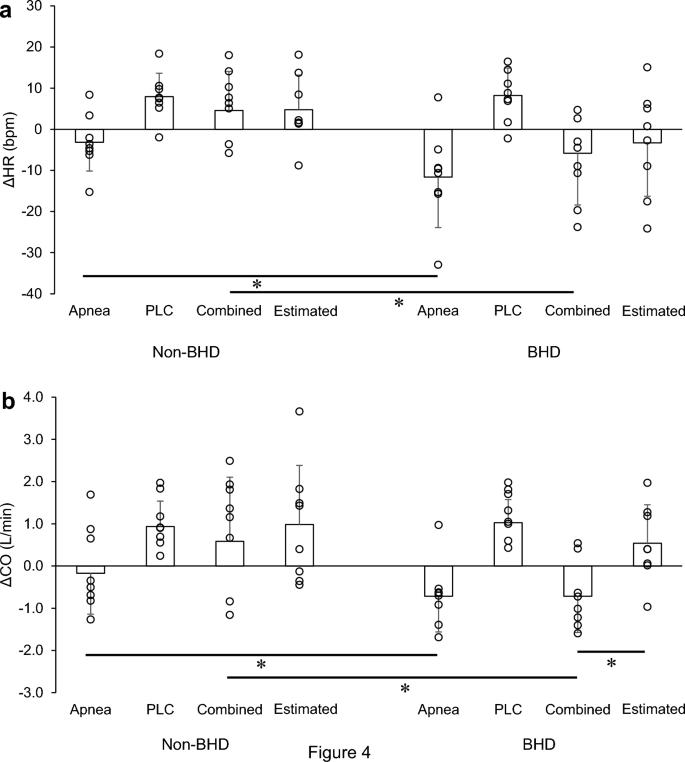
Changes in heart rate ( a ) and cardiac output ( b ) in the apnea, passive leg cycling, and combined trials and the estimated combined trial in the two groups. These data represent individual changes that are underrepresented in Table 2 and Fig. 2 . Jitter plots in each bar show individual’s data. CO cardiac output; HR heart rate; PLC passive leg cycling. Data are presented as mean ± standard deviation. *Significantly different ( P < 0.05)
The major findings in the present study were that, during the combined trial, BHDs showed reductions in their HR and CO, whereas non-BHDs showed increases in these measurements. In the BHD group, ΔHR and ΔCO of the combined trial were similar to those in the apnea trial but not the PLC trial; in turn, in the non-BHD group, the ΔHR and ΔCO of the combined trial were similar to those in the PLC trial, despite similar responses in the apnea and PLC trials in both groups (Figs. 3 , 4 and 5 ). These results suggest that the bradycardia with apnea is prioritized over tachycardia with mechanoreflex solely in BHDs. ΔHR and ΔCO of the combined trial were similar to the summation of responses in the apnea and PLC trials—that is, estimated ΔHR and ΔCO—in non-BHDs, whereas they were significantly different from estimated ΔHR and ΔCO in BHDs. The specific regulation in BHDs may result from adaptation for diving deeper and/or longer.
Cardiovascular responses to apnea and passive leg cycling
Divers showed stronger response to apnea than non-divers. HR and CO were lower in the BHD group than in the non-BHD group (Fig. 3 b and c). Previous studies have shown that bradycardia and peripheral vasoconstriction are greater in BHDs than in non-BHDs (Joulia et al. 2009 ; Peng et al. 2022 ). A strong bradycardia with apnea is considered a consequence of adaptation to diving and it may extend the breath-holding duration (Schagatay and Andersson 1998 ).
Cardiovascular response to apnea is mediated by the autonomic nervous system and includes vagally mediated bradycardia and simultaneous increases in sympathetic mediated peripheral vasoconstriction (Hayashi et al. 1997 ). A previous study indicated that apnea increases cardiac vagal activity (Lemaître et al. 2008 ), a finding which—as the RMSSD of HR is an indicator of cardiac vagal activity (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996 )—is supported by the present result of a greater RMSSD response in the BHD group.
Increases in HR and CO during the PLC trial indicated successful stimulation of the mechanoreflex in both groups (Figs. 2 , 3 f and g). The cardiac response to PLC was probably mainly a consequence of mechanoreflex and of cardiac vagal activity since no obvious voluntary muscular activity was observed and reduction of RMSSD was observed in the present study. Gladwell and Coote ( 2002 ) have suggested that passive stretch selectivity suppresses the cardiac parasympathetic activity and increases HR in human study.
The mechanism behind the different cardiac responses to apnea in BHDs and non-BHDs in the present study is unclear. We can only speculate about the possible role of cardiovagal baroreflex sensitivity (BRS). Because the arterial baroreceptors primarily sense the deformation of the arterial wall rather than intraarterial pressure changes (Aars 1969 ), the cardiovagal BRS is correlated with central arterial mechanical properties such as stiffness and compliance where the baroreceptors are located (the carotid artery and the aortic arch) (Monahan et al. 2001 ; Mattace-Raso et al. 2007 ). Lifelong Japanese female divers demonstrate greater reduction in arterial stiffness than age-matched non-divers (Tanaka et al. 2016 ). The study might suggest that long-term apneic training decreases the arterial stiffness. Thus, BHDs might have had lower arterial stiffness and greater cardiovagal BRS than non-BHDs, resulting in greater bradycardia as a pressor response during apnea. Further studies are needed to investigate the mechanism of how long-term apneic training in BHDs could have contributed to the hemodynamic differences noted compared to those in non-BHDs.
MAP and TPR response to apnea and combined trials were similar between groups (Figs. 3 a, d, 4 a, d). A study suggested that pressor response during apnea is mainly caused by an increase in TPR (Heusser et al. 2009 ). There was a greater increase in muscle sympathetic nerve activity (MSNA) at the end of apnea with FRC in BHDs than in non-BHDs (Breskovic et al. 2011 ). The study also indicated that the augmented sympathetic drive during breath holds was related to chemoreflex stress. FRC breath-hold duration (non-BHD group: 27.7 [22.2–33.2] s, BHD group: 60.4 [34.3–86.5] s) was significantly longer in BHDs, resulting in a higher level of chemoreflex stress. Notably, the slope of the change in total MSNA relative to apnea duration was almost identical between groups, confirming that peripheral and central chemoreflex sensitivity is similar in BHDs and non-BHDs (Dujic et al. 2008 ; Breskovic et al. 2010 ), as shown previously, and that different sympathetic responses between groups were due to variations in apnea duration. If BHDs performed apnea at the FRC for the same duration as non-BHDs, the TPR and MAP responses may not have been different, as in the present study.
Cardiovascular responses to a combination of apnea and mechanoreflex
In the combined trial, the bradycardia with apnea was partially retained in the BHD group, whereas tachycardia was solely observed in the non-BHD group (Fig. 4 b). Similarly, HR and CO during the combined trial were suppressed in the BHD group but increased in the non-BHD group. The estimated combined responses of ΔCO differed from the actual responses in the combined trial in the BHD group (Fig. 5 b). The ΔCO in the combined trial was similar (that is, negative) to that in the apnea trial. These results indicate that the responses are mainly influenced by the inhibitory effect of apnea on the heart solely in BHDs during combined responses.
The difference in the cardiac response to the combined trial between groups was mainly influenced by the response to apnea and/or the combination of PLC and apnea since the response to PLC was similar. The cardiac response of BHDs in the combined trial may indicate vagally mediated bradycardia with apnea in surplus sympathetically mediated tachycardia with PLC. This is partially supported by the RMSSD, which is an index of cardiac parasympathetic activity, that increased in the BHD group in the combined trial while it decreased in the non-BHD group (Table 2 ). It is unclear the mechanisms of how to decide which to prize in the antagonistic regulation between vagally mediated bradycardia with apnea and sympathetically mediated tachycardia with the mechanoreflex.
Limitations
This study had some limitations. First, the generalizability of our findings to other populations is limited because the sample size was small. Second, the present study does not reflect actual diving state such as immersed body. Further studies need to investigate the interaction of apnea and mechanoreflex in real diving condition. Third, we did not evaluate muscle activation across all participants and trials. It is important to conform the absence of active leg cycling during PLC. Fourth, although breathing rate and depth influence HRV analysis (Hirsch and Bishop 1981 ), we did not regulate the respiratory variables; rather, we only assessed short-term RMSSD (30 s). A study showed that recording RMSSD for at least 240 s and 60 s is required to produce good between- and within-day reliability, respectively (Burma et al. 2021 ). Thus, care must be taken in proving the involvement of parasympathetic activity in the present study. Fifth, we did not monitor diaphragmatic oscillation to evaluate involuntary breathing movements (IBM). The apnea at FRC would have led to stronger hypoxemic/hypercapnic stress, concurrently increasing the likelihood of IBM occurring. Previous studies have reported the influence of IBM on hemodynamics (Palada et al. 2008 ; Dujic et al. 2009 ; Stembridge et al. 2017 ).
Conclusions
This is the first study to reveal that the bradycardia with apnea in BHDs is prioritized over tachycardia with the mechanoreflex, whereas the non-BHDs mainly reflects a mechanoreflex-induced response. In the discipline of dynamic apnea, this specific regulation in divers may explain their ability to dive for longer and/or deeper.
Abbreviations
Analog/digital
Analysis of variance
Breath-holding divers
Baroreflex sensitivity
Cardiac output
Electrocardiogram
Electromyography
Exercise pressor reflex
Involuntary breathing movement
Mean arterial pressure
Muscle sympathetic nerve activity
Passive leg cycle
Root mean square of standard deviation of R-R intervals
Stroke volume
Total peripheral resistance
Aars H (1969) Relationship between aortic diameter and aortic baroreceptor activity in normal and hypertensive rabbits. Acta Physiol Scand 75:406–414. https://doi.org/10.1111/j.1748-1716.1969.tb04394.x
Article CAS PubMed Google Scholar
Breskovic T, Valic Z, Lipp A et al (2010) Peripheral chemoreflex regulation of sympathetic vasomotor tone in apnea divers. Clin Auton Res 20:57–63. https://doi.org/10.1007/s10286-009-0034-1
Article PubMed Google Scholar
Breskovic T, Steinback CD, Salmanpour A et al (2011) Recruitment pattern of sympathetic neurons during breath-holding at different lung volumes in apnea divers and controls. Auton Neurosci 164:74–81. https://doi.org/10.1016/j.autneu.2011.05.003
Burma JS, Graver S, Miutz LN et al (2021) The validity and reliability of ultra-short-term heart rate variability parameters and the influence of physiological covariates. J Appl Physiol 130:1848–1867. https://doi.org/10.1152/japplphysiol.00955.2020
Crisafulli A, Marongiu E, Ogoh S (2015) Cardiovascular reflexes activity and their interaction during exercise exercise : general review and functions. BioMed Res Int 2015:1–10. https://doi.org/10.1155/2015/394183
Article CAS Google Scholar
Di Giacomo A, Ghiani GM, Todde F, Tocco F (2021) Cardiovascular responses to simultaneous diving and muscle metaboreflex activation. Front Physiol 12:1–6. https://doi.org/10.3389/fphys.2021.730983
Article Google Scholar
Drew RC, Bell MPD, White MJ (2008) Modulation of spontaneous baroreflex control of heart rate and indexes of vagal tone by passive calf muscle stretch during graded metaboreflex activation in humans. J Appl Physiol 104:716–723. https://doi.org/10.1152/japplphysiol.00956.2007
Drew RC, Blaha CA, Herr MD et al (2017) Muscle mechanoreflex activation via passive calf stretch causes renal vasoconstriction in healthy humans. Am J Physiol Regul Integr Comp Physiol 312:R956–R964. https://doi.org/10.1152/ajpregu.00322.2016
Article PubMed PubMed Central Google Scholar
Dujic Z, Ivancev V, Heusser K et al (2008) Central chemoreflex sensitivity and sympathetic neural outflow in elite breath-hold divers. J Appl Physiol 104:205–211. https://doi.org/10.1152/japplphysiol.00844.2007.-Repeated
Dujic Z, Uglesic L, Breskovic T et al (2009) Involuntary breathing movements improve cerebral oxygenation during apnea struggle phase in elite divers. J Appl Physiol 107:1840–1846. https://doi.org/10.1152/japplphysiol.00334.2009
Ferretti G (2001) Extreme human breath-hold diving. Eur J Appl Physiol 84:254–271. https://doi.org/10.1007/s004210000377
Fico BG, Alhalimi TA, Tanaka H (2022) Vascular responses to simulated breath-hold diving involving multiple reflexes. Am J Physiol Regul Integr Comp Physiol 322:R153–R160. https://doi.org/10.1152/AJPREGU.00202.2021
Gallagher KM, Fadel PJ, Smith SA et al (2006) The interaction of central command and the exercise pressor reflex in mediating baroreflex resetting during exercise in humans. Exp Physiol 91:79–87. https://doi.org/10.1113/expphysiol.2005.032110
Gladwell VF, Coote JH (2002) Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: a role for mechanoreceptors. J Physiol 540:1095–1102. https://doi.org/10.1113/jphysiol.2001.013486
Article CAS PubMed PubMed Central Google Scholar
Hayashi N, Ishihara M, Tanaka A et al (1997) Face immersion increases vagal activity as assessed by heart rate variability. Eur J Appl Physiol Occup Physiol 76:394–399. https://doi.org/10.1007/s004210050267
Heusser K, Dzamonja G, Tank J et al (2009) Cardiovascular regulation during apnea in elite divers. Hypertension 53:719–724. https://doi.org/10.1161/HYPERTENSIONAHA.108.127530
Hirsch JA, Bishop B (1981) Respiratory sinus arrhythmia in humans: How breathing pattern modulates heart rate. Am J Physiol 10:620–629. https://doi.org/10.1152/ajpheart.1981.241.4.h620
Hoffmann U, Smerecnik M, Leyk D, Essfeld O (2005) Cardiovascular responses to apnea during dynamic exercise. Int J Sports Med 26:426–431. https://doi.org/10.1055/s-2004-821113
Ichinose M, Saito M, Kondo N, Nishiyasu T (2006) Time-dependent modulation of arterial baroreflex control of muscle sympathetic nerve activity during isometric exercise in humans. Am J Physiol Heart Circ Physiol 290:1419–1426. https://doi.org/10.1152/ajpheart.00847.2005
Ichinose M, Saito M, Fujii N et al (2008) Modulation of the control of muscle sympathetic nerve activity during incremental leg cycling. J Physiol 586:2753–2766. https://doi.org/10.1113/jphysiol.2007.150060
Ichinose M, Matsumoto M, Fujii N et al (2018) Voluntary apnea during dynamic exercise activates the muscle metaboreflex in humans. Am J Physiol Heart Circ Physiol 314:H434–H442. https://doi.org/10.1152/ajpheart.00367.2017
Joulia F, Lemaitre F, Fontanari P et al (2009) Circulatory effects of apnoea in elite breath-hold divers. Acta Physiol 197:75–82. https://doi.org/10.1111/j.1748-1716.2009.01982.x
Kruse NT, Silette CR, Scheuermann BW (2016) Influence of passive stretch on muscle blood flow, oxygenation and central cardiovascular responses in healthy young males. Am J Physiol Heart Circ Physiol 310:H1210–H1221. https://doi.org/10.1152/ajpheart.00732.2015
Lemaître F, Buchheit M, Joulia F et al (2008) Static apnea effect on heart rate and its variability in elite breath-hold divers. Aviat Space Environ Med 79:99–104. https://doi.org/10.3357/ASEM.2142.2008
Lis A, Łopusiewicz W, Piepoli MF et al (2020) Passive bilateral leg cycling with concomitant regional circulatory occlusion for testing mechanoreflex–metaboreflex interactions in humans. Clin Auton Res 30:549–556. https://doi.org/10.1007/s10286-020-00717-x
Mattace-Raso FUS, Van Den Meiracker AH, Bos WJ et al (2007) Arterial stiffness, cardiovagal baroreflex sensitivity and postural blood pressure changes in older adults: The Rotterdam Study. J Hypertens. https://doi.org/10.1097/HJH.0b013e32811d6a07
Monahan KD, Dinenno FA, Seals DR et al (2001) Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol-Heart Circ Physiol. https://doi.org/10.1152/ajpheart.2001.281.1.H284
Nakamura N, Ikeda N, Heng P, Muraoka I (2022) Muscle stiffening is associated with muscle mechanoreflex-mediated cardioacceleration. Eur J Appl Physiol 122:781–790. https://doi.org/10.1007/s00421-022-04885-8
Nishiyasu T, Tsukamoto R, Kawai K et al (2012) Relationships between the extent of apnea-induced bradycardia and the vascular response in the arm and leg during dynamic two-legged knee extension exercise. Am J Physiol Heart Circ Physiol 302:864–871. https://doi.org/10.1152/ajpheart.00413.2011
Nóbrega ACL, Williamson JW, Friedman DB et al (1994) Cardiovascular responses to active and passive cycling movements. Med Sci Sports Exerc 26:709–714
Palada I, Bakovic D, Valic Z et al (2008) Restoration of hemodynamics in apnea struggle phase in association with involuntary breathing movements. Respir Physiol Neurobiol 161:174–181. https://doi.org/10.1016/j.resp.2008.01.008
Peng H, Oikawa S, Inai Y et al (2022) Effects of lung volume and trigeminal nerve stimulation on diving response in breath-hold divers and non-divers. Respir Physiol Neurobiol 303:103918. https://doi.org/10.1016/j.resp.2022.103918
Schagatay E, Andersson J (1998) Diving response and apneic time in humans. Undersea Hyperb Med 25:13–19
CAS PubMed Google Scholar
Scherrer U, Pryor SL, Bertocci LA, Victor RG (1990) Arterial baroreflex buffering of sympathetic activation during exercise-induced elevations in arterial pressure. J Clin Investig 86:1855–1861. https://doi.org/10.1172/JCI114916
Stembridge M, Hoiland RL, Bain AR et al (2017) Influence of lung volume on the interaction between cardiac output and cerebrovascular regulation during extreme apnoea. Exp Physiol 102:1288–1299. https://doi.org/10.1113/EP086429
Tanaka H, Tomoto T, Kosaki K, Sugawara J (2016) Arterial stiffness of lifelong Japanese female pearl divers. Am J Physiol Regul Integr Comp Physiol 310:975–978. https://doi.org/10.1152/ajpregu.00048.2016.-Japanese
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. task force of the european society of cardiology and The North American Society of Pacing and Electrophysiology. Circulation 93:1043–1065
Tocco F, Crisafulli A, Melis F et al (2012) Cardiovascular adjustments in breath-hold diving: Comparison between divers and non-divers in simulated dynamic apnoea. Eur J Appl Physiol 112:543–554. https://doi.org/10.1007/s00421-011-2006-0
Tokizawa K, Mizuno M, Nakamura Y, Muraoka I (2004a) Venous occlusion to the lower limb attenuates vasoconstriction in the nonexercised limb during posthandgrip muscle ischemia. J Appl Physiol 96:981–984. https://doi.org/10.1152/japplphysiol.00695.2003
Tokizawa K, Mizuno M, Nakamura Y, Muraoka I (2004b) Passive triceps surae stretch inhibits vasoconstriction in the nonexercised limb during posthandgrip muscle ischemia. J Appl Physiol 97:1681–1685. https://doi.org/10.1152/japplphysiol.00312.2004
Vestergaard MB, Larsson HBW (2019) Cerebral metabolism and vascular reactivity during breath-hold and hypoxic challenge in freedivers and healthy controls. J Cereb Blood Flow Metab 39:834–848. https://doi.org/10.1177/0271678X17737909
Wesseling KH, Jansen JRC, Settels JJ, Schreuder JJ (1993) Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 74:2566–2573. https://doi.org/10.1152/jappl.1993.74.5.2566
Download references
Acknowledgements
The authors appreciate the support received from all participants in this study. Moreover, they would like to thank Editage ( www.editage.com ) for English language editing.
Author information
Authors and affiliations.
Faculty of Sport Sciences, Waseda University, 2-579-15 Mikajima, Tokorozawa, Saitama, 359-1192, Japan
Nakamura Nobuhiro & Hayashi Naoyuki
Graduate School of Sport Sciences, Waseda University, Tokorozawa, Saitama, Japan
You can also search for this author in PubMed Google Scholar
Contributions
All authors conceived and designed the study. NN and PH conducted experiments. NN analyzed the data. All authors interpreted the data. NN wrote the first manuscript and NN and NH edited the manuscript.
Corresponding author
Correspondence to Hayashi Naoyuki .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflicts of interest, financial or otherwise.
Additional information
Communicated by Guido Ferretti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Nobuhiro, N., Heng, P. & Naoyuki, H. The interaction of breath holding and muscle mechanoreflex on cardiovascular responses in breath-hold divers and non-breath-hold divers. Eur J Appl Physiol 124 , 2183–2192 (2024). https://doi.org/10.1007/s00421-024-05431-4
Download citation
Received : 12 October 2023
Accepted : 30 January 2024
Published : 05 March 2024
Issue Date : July 2024
DOI : https://doi.org/10.1007/s00421-024-05431-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Muscle mechanoreflex
- Cardiovascular response
- Find a journal
- Publish with us
- Track your research

IMAGES
COMMENTS
Nov 25, 2020 · Within about ten seconds after starting the breath hold, heart rate is dropping. It ends up decreasing by 27 beats per minute, reaching its low point after 83 seconds on average. This is fairly ...
Throughout the first breath-holding, heart rate (HR) increased from 89.5 ± 3.9 bpm to 107.6 ± 4.2 . bpm (P <0.05). The ECG JT interval decreased at the onset of breath-holdings, the intervals ratio (JT/RR) increased (P <0.05), and the ST-segment depression was not altered significantly. Arterial blood flow (ABF) was reduced from 3.5
Breathing Technique Heart Rate (bpm) Mean Breath Rate (bpm) Max Min ∆ Mean Rest _____ Shallow Abdom. Bellows Deep Abdom. Exercise 2: Apnea and Heart Rate Aim: To determine the effect of apnea on the subject’s heart rate by having the subject hold his or her breath. Procedure 1. The subject should sit quietly and breath normally before the ...
The aim of the present study was to analyze the cardiac responses to prolonged breath-holding in elite divers during a competition. Heart rate behaviour and the incidence of arrhythmia were recorded in 16 well-trained breath-hold divers (BHD) using a cardio-frequency meter (for 15 divers) and a Holter (for one diver) during maximal static ...
6. Enter the subject’s resting heart rate and breathing rate values into Table 1. Exercise 2: Heart Rate and Apnea Aim: To measure the heart rate of a subject while they are holding their breath. Approximate Time: 15 minutes Procedure 1. Click on the Record button. The signal should begin scrolling across the screen. 2.
The heart rate right after breath holding increased in both conditions (78.08 BPM in cold dive control and 56.87 BPM in cold dive condition). Compared to the percent difference from heart rate at rest, the mean heart rate decreased by 9% during the last 15 seconds of the cold dive control breath holding. In contrast, the mean heart rate
The goal of this study was to investigate the effect of repetitive end-inspiration breath holding on very short-term HRV. A total of 32 young healthy participants took part in the experiments. Three trials were performed, each involving seven repetitive end-inspiration breath holding and a 30 s recovery period between breath holding.
Breathing. In this experiment, you will record breathing movements with a respiratory belt transducer fastened around the abdomen. You will investigate various aspects of breathing, including the ability to hold the breath, hyperventilation, rebreathing, and the relation between breathing and heart rate.
Figure HH-5-L4: Heart rate change during apnea. Questions 1. How does the subject’s average heart rate while resting compare to the heart rate while holding breath? 2. How do the subject’s maximum and minimum heart rates while resting compare to the same heart rates while holding breath? Exercise 3: Heart Rate While Testing the Diving Reflex
Mar 5, 2024 · Cardiovascular responses to breath holding are characterized by bradycardia and peripheral vasoconstriction (Lemaître et al. 2008).This response plays an important role in preserving the oxygen supply to vital organs such as the heart and brain (Nishiyasu et al. 2012; Vestergaard and Larsson 2019) and cardiovascular responses induced by the breath-holding reflex may extend the maximal apnea ...