- High School
- You don't have any recent items yet.
- You don't have any courses yet.
- You don't have any books yet.
- You don't have any Studylists yet.
- Information

Exp. 11 Lab Report
General chemistry ii (chem 1110 ), california state university, los angeles, recommended for you.
Students also viewed
- Lab Experiment #21 - Lab report The Thermodynamics of the Borax Solubility
- Equilibrium and Le Chatlier's Principle Lab Report
- Exp. 17 Borax
- Exp. Equil. Handout - Lab Report
- Prelab - Forgot
- Experiment #17 Formula and Formation Constant of a Complex
Related documents
- pH and pH Titrations
- Hydrolysis of t-Butyl Chloride
- Ex P 24 Synth Sodium Chem 1110
- Expediente Grupo 3 - La narracion
- Qualatative analysis of ions by paper microfluidics
- Prelab Synthesis & Analysis of Sodium xx Hy
Related Studylists
Preview text.
Experiment 11. Synthesis of Benzoic Acid
Purpose: The purpose of this experiment is to prepare benzoic acid through oxidation. Then our benzoic acid will be recrystallized and eventually will be analyzed by a pH titration with standard NaOH in a later experiment. Theory/Principles: Benzaldehyde under a mild oxidizing condition in a basic solution oxidizes the –CHO group to – CO2 while the rest of the molecule remains unchanged. In this experiment potassium permanganate is used to oxidize benzaldehyde giving the reaction of C6H5CHO + MnO^- 4 + OH- C6H5COO- + MnO2 + H2O. In order to create neutral benzoic acid then an a strong acid (ethanol) is added to our solution giving the reaction of C6H5COO- (aq) + H+ (aq) C6H5COOH. Since benzoic acid is soluble in water at room temperature then we must add HCl to precipitate it until solution is acid. However, recrystallization will occur when benzoic acid is dissolved in hot water and the solution is cooled. Through recrystallization benzoic acid is purified. Leaving the final product over for the next lab period will completely dry our product in which we will weigh it in order to obtain the % yield of benzoic acid based on our collected mass of benzaldehyde we used. To obtain the % yield we are given the equation of: observed mass of product/ maximum possible mass of product x 100%. Experimental Procedures: - No changes done to lab experiment procedure except for the procedure lasting 3 labs due to having to leave our recrystallized product to fully dry in order to gain its mass Goldwhite, H.; Tikkanen, W. Experiment 11 of Benzoic Acid, Experiments in General Chemistry, 4th ed.;The McGraw Hill Companies. (78-79)
Data Tables/Summary:
- Data # 2: Formatted version of my data collected from the lab experiment including extra calculated data such as mass of recrystallized benzoic acid & calculated % yield of benzoic acid Mass of 10 mL benzaldehyde (g):cylinder + 30 g Mass of 10 mL cylinder + benzaldehyde after transfer (g): 24 g Mass of watchglass used for benzoic acid (g): 53 g Mass of recrystallized benzoic acid + watchglass (g): 56 g Mass of benzaldehyde used (g): 5 g Mass of recrystallized benzoic acid (g): 2 g
% Yield of Benzoic Acid: 50%
Results and Discussion:
Sample Calculations:
Calculating Mass of benzaldehyde= (mass of cylinder + benzaldehyde – mass of cylinder + benzaldehyde after transfer): (30.0g-24 g)= 5 g
Calculating Mass of recrystallized benzoic acid= (mass of recrystallized benzoic acid + watch glass – mass of watch glass): (56 g- 53 g)= 2 g
Calculating Theoretical Mass of Benzoic Acid: Mass of C7H6O (Benzaldehyde) = 106 g/mol
1:1 ratio 0 moles x 1 mol. of benzoic acid/ 1 mol. of benzaldehyde= 0 moles Mass of C7H6O2 (Benzoic Acid) = 122 g/mol 0 moles x 122 g/ mol. = 5 g of benzoic acid
Calculating % yield of Benzoic acid:
% yield= (2 g / 5 g) x 100 %= 50 % yield of benzoic acid
Logical Explanation:
By going through the process of synthesizing benzoic acid I was able to obtain the necessary data needed for determining my % yield of benzoic acid and creating a recrystallized product that I would eventually need for a later experiment. In order to reach to my final calculation of 50% yield of benzoic acid I needed the specific data of 10 mL grad. cylinder + benzaldehyde and the mass of 10 mL grad. cylinder + benzaldehyde after transferring it into my solution of KMnO4/NaOH in order to obtain my mass of benzaldehyde used in grams which I would eventually use for finding the number of moles of benzaldehyde by dividing my grams of
90 % yield ammonia: 1 x 10^7 g x 100/90 = 1 x 10^7 g
1 ton= 907185 g
Tons of H2 required: 1 x 10^7 g/ 907185 = 21 tons
- During the oxidation of benzaldehyde with KMnO4/NaOH I saw color changes from initially purple to turning dirty brown due to MnO2 forming as the reaction progresses. By adding the remaining portions of KMnO4 to the solution the color purple of KMnO4 will eventually fade to it just being a really dark brown color. Thus, making the inorganic compounds KMnO4/NaOH and MnO2 responsible for the colors observed in the experiment.
Conclusion:
Through the preparation of benzoic acid through oxidation in order to obtain a purified recrystallized product and collect the data necessary along the way I was able to find my % yield of benzoic acid of 50% at the end of the experiment.
References:
Goldwhite, H.; Tikkanen, W. Experiment 11 of Benzoic Acid, Experiments in General Chemistry, 4th ed.;The McGraw Hill Companies. (77-79)
- Multiple Choice
Course : General Chemistry II (CHEM 1110 )
University : california state university, los angeles.

- Discover more from: General Chemistry II CHEM 1110 California State University, Los Angeles 122 Documents Go to course
- More from: General Chemistry II CHEM 1110 California State University, Los Angeles 122 Documents Go to course
- More from: CH110 LABS winter/fall by no one 9 9 documents Go to Studylist
Preparation and Analysis of Benzoic Acid
The purpose of this experiment is to prepare and analyze benzoic acid. It is also meant to demonstrate some general preparation methods, such as using a reflux apparatus, vacuum filter, and a hot gravity filter, plus determining melting point and titrating.
Introduction
Benzoic acid was first synthesized in the 16th century [W2009] . It is an organic acid that naturally forms monoclinic leaflets and is found as a colourless solid. The molar mass of benzoic acid is 122.12 g and its melting point is 122°C. In water at 1°C, benzoic acid has a solubility of 1.7 g/liter, but at 95°C it has a solubility of 68 g/liter [ND2008] . Due to its natural ability to impede the growth of yeast, mold, and some bacteria, benzoic acid has been used to treat fungal skin diseases such as athlete's foot and as a food preservative.
Oxidation Theory and Calculations
In general, a primary alcohol can be oxidized to lend either aldehydes or carboxylic acids [JC2006] . The reaction goes through two processes. Note that R is shorthand for an arbitrary hydrocarbon counterpart. For example, RCH 2 OH would be ethanol if R was CH 3 .
RCH 2 OH + O- ⇋ RCOH + H 2 O
RCOH + O- ⇋ RCOOH
In the first stage, an available oxygen atom binds with two of the hydrogen atoms at the end of the alcohol (RCH 2 OH) to produce an aldehyde (RCOH). The oxygen within the alcohol then assumes a covalent configuration with the carbon and the result is a molecule known as an aldehyde. In the second stage, the hydrogen atom at the end of the aldehyde incorporates another available oxygen to produce a carboxylic acid (RCOOH). This reaction has several uses. For example, potassium dichromate (an oxidant) will produce ethanal or ethanoic acid when mixed with ethanol. This particular reaction used to be used as part of the ‘breathalyser’ procedure to test if drivers had consumed too much alcohol. In our particular experiment, we are oxidizing benzyl alcohol (C 6 H 5 CH 2 OH) with permanganate (MnO 4 - ). The goal is to produce benzoic acid (C 6 H 5 COOH). Thus, in our case the R will be C 6 H 5 , a phenyl ring. The permanganate is created by dissolving potassium permanganate in sulphuric acid (H 2 SO 4 ) (the reason for using a highly acidic solvent will be explained later). To understand how the reaction works, we must first understand how the oxidation process works. Oxidation processes are described using redox equation. Redox equations describe the requirements for changing the oxidation level of a chemical. First, consider the permanganate. It reacts in two steps: first to MnO 2 then Mn 2+ . Their redox equation [VB1986] is written as:
MnO 4 - + 4 H + + 3 e - ⇋ MnO 2 + 2 H 2 O
MnO 2 + 4 H + + 2 e - ⇋ Mn 2 + + 2 H 2 O
And for the two states of the alcohol to acid reaction:
RCH 2 OH ⇋ RCOH + 2 H + + 2 e -
RCOH + H 2 O ⇋ RCOOH + 2 H + + 2 e -
These equations are not complete since they have spare electrons on one side (because of this they are often called half-equations). Note, however, how equations 3 and 4, and 5 and 6 have spare electrons on the opposite sides. This is because 3 and 4 are reduction while 5 and 6 are oxidation. These equations can be combined to produce the full equations for the oxidation. For example, combine 3, 4, and 5 to determine the alcohol to aldehyde step:
3 RCH 2 OH + 2 MnO 4 - + 2 H + ⇋ 3 RCOH + 2 MnO 2 + 4 H 2 O
RCH 2 OH + MnO 2 + 2 H + ⇋ RCOH + Mn 2+ + 2 H 2 O
5 RCH 2 OH + 2 MnO 4 - + 6 H + ⇋ 5 RCOH + 2 Mn 2+ + 8 H 2 O
Equation 7 is the combination of 3 and 5, the first step of manganese's oxidation; 7 is from 4 and 5, the second step of the oxidation; and 9 is the combination of 7 and 8. The same can be done to determine the aldehyde to alcohol step:
3 RCOH + 2 MnO 4 - + 2 H + ⇋ 3 RCOOH + 2 MnO 2 + H 2 O
RCOH + MnO 2 + 2 H + ⇋ RCOOH + Mn 2+ + H 2 O
5 RCOH + 2 MnO 4 - + 6 H + ⇋ 5 RCOOH + 2 Mn 2+ + 3 H 2 O
Finally, equations 9 and 12 can be combined to produce the equation for the overall reation (assuming it goes to completion):
5 RCH 2 OH + 4 MnO 4 - + 12 H + ⇋ 5 RCOOH + 4 Mn 2+ + 11 H 2 O
Replacing R with C 6 H 5 gives the reaction for the benzyl chemicals.
5 C 6 H 5 CH 2 OH + 4 MnO 4 - + 12 H + ⇋ 5 C 6 H 5 COOH + 4 Mn 2+ + 11 H 2 O
Experimental Procedure
Benzyl alcohol (4 ml) and KMnO 4 (about 9 g) are placed into 150 ml of a highly acidic solution (H 2 SO 4 ). Once in the solution, the KMnO 4 will dissolve into K + and MnO 4 - . There are about twice as many moles of KMnO 4 to encourage the alcohol to react as far as possible. As the amount of benzyl alcohol approaches or exceeds the 5/4 of the amount of KMnO 4 , there will be significant amounts of benzylhyde left over. Also, the solution needs to be acidic so that it will supply the free hydrogen ions that appear on the left-hand side of equation 14.
In order to push the equilibrium to the right as quickly as possible, the solution is heated in a ball flask and stirred over a magnetic stirring plate. Unfortunately, as the solution heats up, the solution will start to evaporate. Benzaldehyde has the lowest boiling point (178.1°C) and will evaporate first. Benzaldehyde has a strong smell of vanilla, so it should be relatively noticeable when it starts to evaporate. The benzaldehyde is an intermediate chemical in the reaction and if it evaporates, the final yield will be reduced. To prevent this, the solution is placed in a reflux apparatus while the heating is taking place.
Apart from simply increasing the activity of the molecules, increasing the reaction. It also helps the oxidation process since KMnO 4 decomposition is sensitive to heat.
The reaction takes a long time to complete, so the procedure is stopped part way through. As a result, the solution will still contain MnO 4 - and MnO 2 . These are reduced by adding sodium bisulphite (the MnO 4 - and MnO 2 oxidize the bisulphite reacts with these to produce manganese, water, and sulphate ions, see equations 15 and 16). The result of this should be noticeable as the brownish color of manganese dioxide will fade. Not all of the MnO 4 - and MnO 2 will be removed at this point, so some color will persist.
2 MnO 4 - + 4 HSO3- + 2 H + ⇋ 2 Mn 2+ + 5 SO 4 2- + 3 H 2 O
MnO 2 + HSO3- + H + ⇋ Mn 2+ + SO 4 2- + H 2 O
The benzoic acid is slowly cooled. It freezes at 122.4°C, creating crystals. The crystals are removed by vacuum filtration, and tests are run on them.
After this, the benzoic acid crystals are dissolved in water for further purification. Since MnO 2 and Mn 2+ are not soluble in water, they are easily removed via gravity filtration. Then the acid is split into two beakers: one hot, and one cool. These are then cooled so that the acid will recrystallize. Note that unless the solution is cooled ahead of time, the part of the solution that is place in the cool beaker will cool down very rapidly. This hinders the crystallization process because it produces large amounts of very small crystals.
The crystals are separated from the solution again and the final tests are made.

Testing Procedures
The effectiveness of the procedure is measured by 3 different tests. First, the crude acid crystals are weighed and compared against the expected yield to determine the % conversion. Second, some of the acid crystals are melted to test the accuracy of the melting point (ideally 122°C). Third, some of the purified benzoic acid is redissolved in water and titrated against sodium hydroxide (NaOH). The moles of NaOH needed for the titration equals the moles of benzoic acid, which is then converted to grams, and can be compared against the actual mass of benzoic acid produced to determine the purity of the product.
Observations
During reflux in the first trial the solution changed colour several times. First, it changed from dark purple to orange, then to light yellow, then to dark yellow, then burnt orange, and finally a brownish orange. 0.012 g of dullish light yellow crystals was retrieved, which was very unfortunate because 0.1 g was needed for determining the melting point and ~0.15 g to determine the purity through three titrations. Much of the loss of crystals was during reflux when huge amounts evaporated because the solution was too hot. During the second trial the experiment was done more carefully and only a few vapours were released when refluxation first started. Ten minutes into the procedure, the solution turned a pinkish colour, then light yellow, then darker-medium yellow at 15 minutes, and at 30 minutes the solution was bright yellow with a thin orange layer floating on the top. After vacuum filtration there was 1.509 g of light brown crystals. Two samples of the filtered crystals were put into beakers, one pre-warmed and one cooled in an ice bath. The latter crystals formed faster, cleaner, and smaller. Whereas the others, left at room temperature, formed slower, but were bigger. When the crystals on filter paper were examined, the cold crystals were off-white and very fine with a paper-like texture, and the warm crystals were coarse, off-white/pearly, and very bumpy. The average melting point for the first trial was 94 degrees Celsius, and 115 degrees Celsius for the second trial. The normal melting point of benzoic acid is 122.4 degrees. The purity of the crystals was measured using titration with sodium hydroxide as the titrant.
Calculations for Percent Yield
Titration calculations, titration #1, titration #2.
In the first trial run of this experiment, 0.12 g of crystals was obtained. These crude benzoic acid crystals were a dull yellow colour and they didn’t look very pure. The low yield, 2.57%, was a result of several errors. First of all, the 150 mL of H 2 SO 4 was measured with a 400 mL beaker which is not very precise. Also, when the original solution was refluxed a large amount of fumes escaped through the top of the reflux apparatus without condensing. This was because the heat was turned on too high, and so some of the crystals evaporated, thereby reducing the yield. Also once the solution was finished refluxing, the solution was a brownish-orange colour and therefore the crystals were a dullish yellow colour versus being pure and white. Because the crystals were not very pure, the melting point was only found to be 94°C, whereas benzoic acid is supposed to have a melting point of 122°C. After recrystallizing and purifying the crude benzoic acid crystals, the cold crystal solution sat in the fridge and the room temperature crystal solution sat in the lab for a week. Then, because no crystals had formed over the week, the experiment was discontinued and therefore neither the titrations nor the percent purity calculations were completed. The lack of crystals was due to the fact that there was a low percent yield of 2.57%, and the hot gravity filtration apparatus was not completely hot when the crystals were filtered through.
The second trial run was more successful because 1.509 g of crude benzoic acid crystals was collected. The crude crystals were light brown with some clear spots. There was less error in this experiment because the 150 mL of H 2 SO 4 was measured with a 10 mL graduated cylinder and care was taken to not release vapors during refluxation. Although some vapors did escape, it was considerably less than in the first trial run. The crude product resulted in a 32.3% yield. We obtained 1.509 g of crystals compared to the theoretical amount of 4.67 g. The melting point of these crude crystals was 115°C which is very close to the actual melting point of benzoic acid: 122°C. Once the crude crystals were recrystallized, purified, and separated into two beakers of different temperature, it was discovered that the cold crystals formed faster, yet were smaller, than the ones that formed at room temperature. The 0.216 g of cold crystals were very fine and off-white compared to the 0.173 g of lukewarm crystals which were coarse and had an off-white, pearly appearance. In the experiment, the melting point of the pure crystals was not determined, so it cannot be compared with the melting point of the crude crystals. Because not a lot of pure crystals were obtained, the titration was only completed twice although it should have been done three times. The first titration of the room temperature crystals determined that the crystals had 49.1% purity. The second titration, which was of the cold pure crystals, determined a purity of 268%. Obviously this percentage is inaccurate and that is due to a faulty titration. Phenolphthalein was not added to the solution and therefore the titration was off. When the phenolphthalein was added, the solution was already bright pink and the titration was well beyond the equivalence point.
In this experiment benzoic acid was synthesized through the oxidation of benzyl alcohol by acidified potassium permanganate.
The alteration of our initial solution into crude crystals was observed through using a reflux apparatus, collecting and cleaning the crystals with a vacuum filter, recrystallizing again through reflux, and finally purifying with a hot gravity filter. The melting point was examined, the percent yield calculated and the purification of the resulting crystals was discovered through titration with phenolphthalein. Although the first trial was not successful, the second trial resulted in a percent yield of crude crystals of 32.1%, a melting point of 115°C, and a percent purity of 49.1%.
Chemistry Online
Synthesis of benzoic acid from Grignard reagent
Written by J.A Dobado | Last Updated on April 22, 2024
Students will familiarized with the strict anhydrous conditions involved in the use of Grignard reagents , and in this case for obtaining benzoic acid by the carbonation reaction of a Grignard reagents .
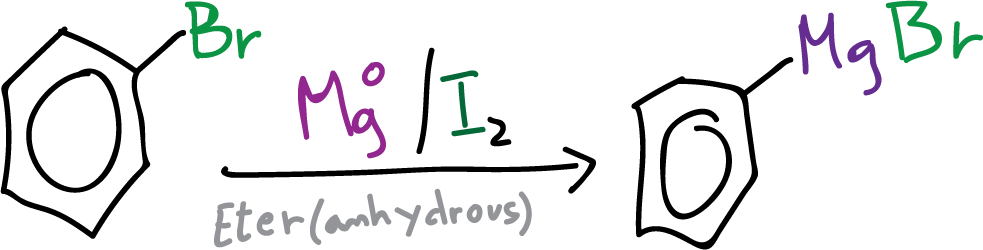
One of the most popular methods of forming C-C bonds in organic synthesis is through the use of Grignard reagents ( Nobel Prize in Chemistry for 1912 ). The formation of these organometallic compounds involves the reaction of an alkyl, vinyl or aryl halide with magnesium and involves a change in the electronic nature of the carbon atom, which changes from electrophile in the halide to strongly nucleophile in the organomagnesium compounds.
R- X + Mg → R-Mg X
X = Cl, Br, I
Grignard compounds are highly reactive, they react with water, oxygen, CO 2 , etc. Therefore, these compounds must be prepared under anhydrous conditions using an inert atmosphere . They have a strongly nucleophilic (and basic) character and produce additions to carbonyl groups, with the formation of a new C-C bond, giving alcohols whose nature depends on the type of carbonyl starting compound used.
A Grignard reagent is considered to be a carbon or carbanion bond (the magnesium salt of an acidic hydrocarbon). However, it is more accurate to consider Grignard reagents as having a highly polar covalent C-Mg bond, rather than an ionic bond between C ⊖ and ⊕ MgX.
In this experiment, we will proceed to synthesize benzoic acid from bromobenzene by converting the latter into a Grignard reagent ( Ph-MgX ). The Grignard reagent is then reacted with gaseous carbon dioxide (CO 2 ) to form benzoic acid .
The Grignard synthesis of a carboxylic acid (R-COOH) is achieved by a carbonization step by bubbling carbon dioxide , gaseous CO 2 , into a solution in THF or diethyl ether of the reagent. In our case, ground dry ice (solid CO 2 ) is used over the Grignard reagent . The advantage of using dry ice is that it acts not only as a reagent but also as a cooling agent.
The reaction proceeds through the formation of an intermediate carboxylate salt, which is then protonated to yield benzoic acid.
In conclusion, the synthesis of benzoic acid from a Grignard reagent is a useful method for the preparation of carboxylic acids. The reaction is also highly selective, with no other major products formed and the yield of the reaction was 80%, which is a good yield for this type of reaction.
Experimental procedure
A) preparation of grignard reagent (phenylmagnesium bromide).
In order to carry out this reaction successfully, it is necessary that both the reagents and the material used are completely dry (use an oven for the material if necessary) and work under inert atmosphere conditions.
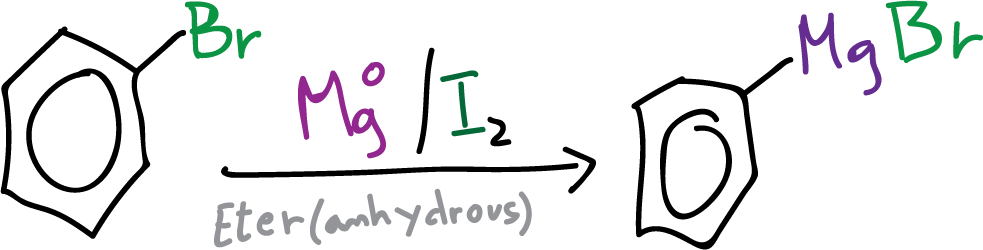
In a 250 ml round bottom flask equipped with a reflux condenser, and an addition funnel, under inert argon atmosphere, 2.4 g of magnesium Mg (in the form of chips or filings) are placed. Subsequently, 30 ml of diethyl ether (anhydrous) are added (THF can also be used).
A small amount of activating agent (usually dibromoethane or iodine ) is added and once the magnesium surface is activated (traces of iodine are added to start the reaction at low temperatures without having to resort to an initial heating, since the reaction is then exothermic), slowly add 10 ml of anhydrous bromobenzene .
If the reaction is not started immediately, it is heated in a water bath and removed when the solution begins to boil (discoloration of the iodine and appearance of turbidity is observed).
Since the reaction is exothermic, gentle boiling is allowed to proceed for 30-40 min. At this point, the heating is cut off to control the reaction (because it is exothermic) until the formation of phenylmagnesium bromide .
B) Grignard reagent carbonation
In a beaker of 250 ml, 15.14 g dry crushed ice ( solid CO 2 ) is placed. The phenylmagnesium bromide solution prepared in the previous step is poured slowly over it, using magnetic agitation.
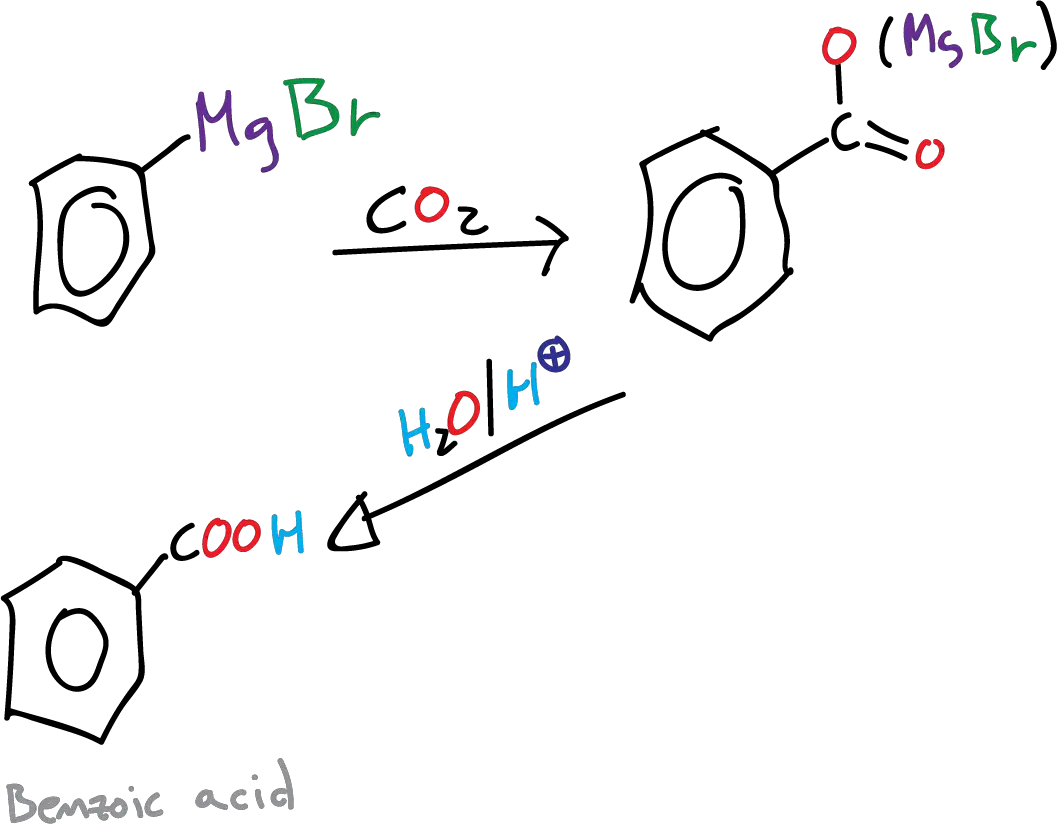
A pasty mass is obtained, which is continued stirring until all the solid CO 2 has sublimated. Then, 50 ml of hot water are added, and it is acidified with HCl (dil.), for this way to be able to dissolve the phenylmagnesium salt and to obtain by precipitation the benzoic acid . It is cooled with an ice bath and filtered under vacuum . The benzoic acid thus obtained can be recrystallized from water.
Physico-chemical properties
This table collects data for the molecular weight (M w ), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals ( GHS ) and are collected in the followinf Table for the chemical compounds used in this experiment.
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
Video on the synthesis of benzoic acid from Grignard reagent
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. ( 2015 ). Experimental Organic Chemistry Laboratory Manual . Elsevier Science & Technology . ISBN: 978-0-12-803893-2
- A Three-Step Synthesis of Benzoyl Peroxide Brenda Her, Alexandra Jones, and James W. Wollack Journal of Chemical Education 2014 91 (9), 1491-1494 DOI: 10.1021/ed400240k
Return to the Organic Synthesis Experiments.

IMAGES
COMMENTS
Through the preparation of benzoic acid through oxidation in order to obtain a purified recrystallized product and collect the data necessary along the way I was able to find my % yield of benzoic acid of 50% at the end of the experiment. References: Goldwhite, H.; Tikkanen, W. Experiment 11 of Benzoic Acid, Experiments in General Chemistry ...
Learn how to prepare benzoic acid from bromobenzene using the Grignard reaction, a method for forming carbon-carbon bonds. Follow the experimental procedure, pre-lab preparation and post-lab analysis of the reaction products.
Learn how to prepare and analyze benzoic acid by oxidizing benzyl alcohol with permanganate. The web page explains the theory, calculations, experimental procedure, and results of the experiment.
Crude sample of benzoic acid,250 ml measuring flask,funnel,a glass rod,and a trough. Procedure 1.take about 2-3ml of the crude sample of benzoic acidin a 250ml beaker,in another take about 150ml of water and keep it for boiling. 2.add slowly with stirring least amount of boiling water to the beaker containing crude sample of benzoic acid so ...
Learn how to use vitamin B1, thiamine, as a coenzyme to convert benzaldehyde into benzoin in a multi-step synthesis. Explore the biochemical and organic chemistry aspects of this reaction and design your own experiment to produce benzoic acid.
Learn how to prepare benzoic acid by carbonating a Grignard reagent derived from bromobenzene under anhydrous conditions. The experiment involves the formation of phenylmagnesium bromide, the reaction with dry ice, and the acidification and filtration of the product.
Learn how to synthesise benzoic acid using bromobenzene, magnesium metal and dry ice in this experiment. Follow the steps of preparing and working with Grignard reagents, the reaction with CO2, and the work-up and purification of the product.
§Experiment 4 Synthesis of Benzoic Acid Objectives • To produce the Grignard reagent in a water free environment. • To react the Grignard reagent with dry ice, CO 2(s). • To assess the purity of the product by determining its melting point. • To determine the molar mass of the product via titration with standardized NaOH. In the Lab • Students work in pairs
Preparation of Benzoic Acid using the Grignard Reaction. In this experiment, the alkyl magnesium halide will be in the form of phenyl magnesium bromide (R = C 6 H 5 in eq. 1), which you will prepare from bromobenzene. The phenyl magnesium bromide will be quenched with solid carbon dioxide (eq. 3) and then benzoic acid is isolated from the ...
Place the benzoic acid in a clean, labeled vial. Take a melting point. Take an IR of the product using a cast film technique as described in the lab textbook. Report See the lab manual sections Laboratory Reports and Synthesis Reports for the format for submitting your report for this experiment. Include a Separation Scheme and marked up