Controlled Experiment
Saul McLeod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul McLeod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Learn about our Editorial Process
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
This is when a hypothesis is scientifically tested.
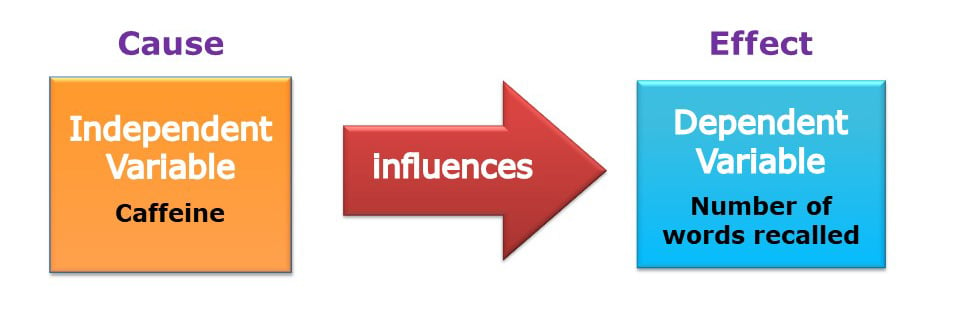
In a controlled experiment, an independent variable (the cause) is systematically manipulated, and the dependent variable (the effect) is measured; any extraneous variables are controlled.
The researcher can operationalize (i.e., define) the studied variables so they can be objectively measured. The quantitative data can be analyzed to see if there is a difference between the experimental and control groups.

What is the control group?
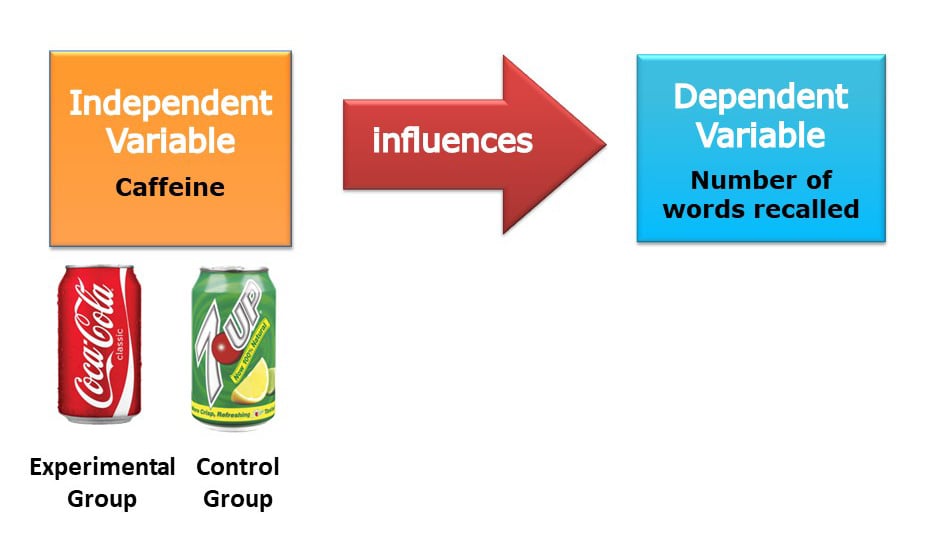
In experiments scientists compare a control group and an experimental group that are identical in all respects, except for one difference – experimental manipulation.
Unlike the experimental group, the control group is not exposed to the independent variable under investigation and so provides a baseline against which any changes in the experimental group can be compared.
Since experimental manipulation is the only difference between the experimental and control groups, we can be sure that any differences between the two are due to experimental manipulation rather than chance.
Randomly allocating participants to independent variable groups means that all participants should have an equal chance of participating in each condition.
The principle of random allocation is to avoid bias in how the experiment is carried out and limit the effects of participant variables.
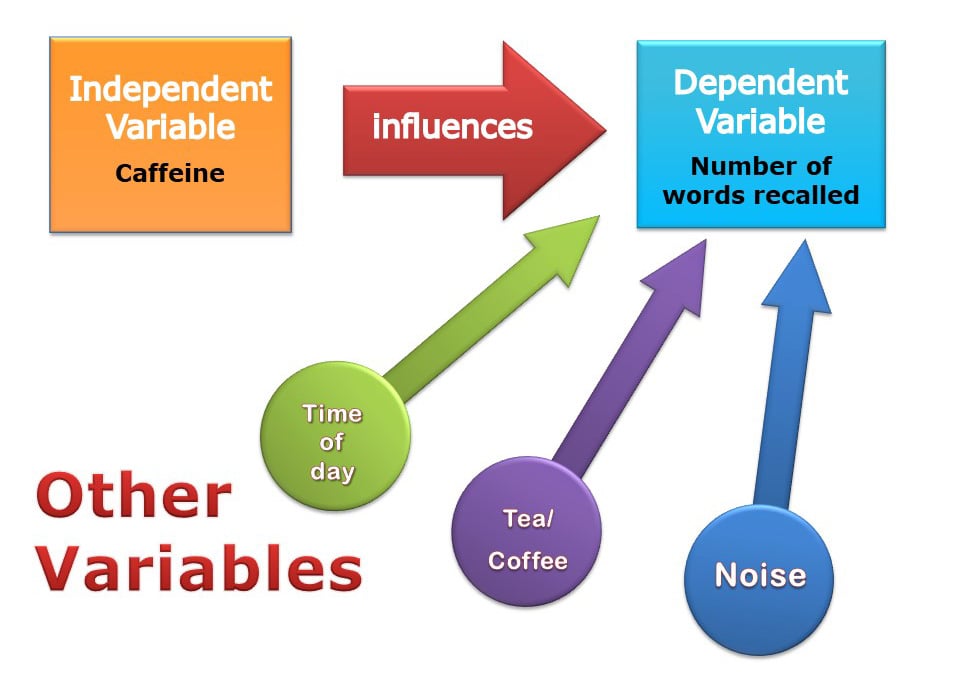
What are extraneous variables?
The researcher wants to ensure that the manipulation of the independent variable has changed the changes in the dependent variable.
Hence, all the other variables that could affect the dependent variable to change must be controlled. These other variables are called extraneous or confounding variables.
Extraneous variables should be controlled were possible, as they might be important enough to provide alternative explanations for the effects.
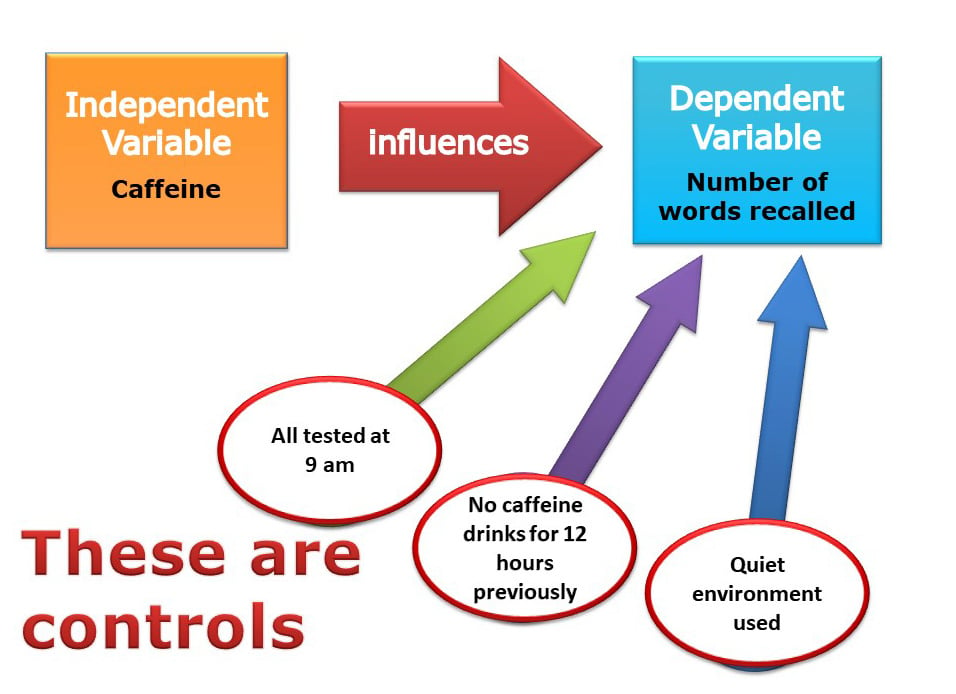
In practice, it would be difficult to control all the variables in a child’s educational achievement. For example, it would be difficult to control variables that have happened in the past.
A researcher can only control the current environment of participants, such as time of day and noise levels.
Why conduct controlled experiments?
Scientists use controlled experiments because they allow for precise control of extraneous and independent variables. This allows a cause-and-effect relationship to be established.
Controlled experiments also follow a standardized step-by-step procedure. This makes it easy for another researcher to replicate the study.
Key Terminology
Experimental group.
The group being treated or otherwise manipulated for the sake of the experiment.
Control Group
They receive no treatment and are used as a comparison group.
Ecological validity
The degree to which an investigation represents real-life experiences.
Experimenter effects
These are the ways that the experimenter can accidentally influence the participant through their appearance or behavior.
Demand characteristics
The clues in an experiment lead the participants to think they know what the researcher is looking for (e.g., the experimenter’s body language).
Independent variable (IV)
The variable the experimenter manipulates (i.e., changes) – is assumed to have a direct effect on the dependent variable.
Dependent variable (DV)
Variable the experimenter measures. This is the outcome (i.e., the result) of a study.
Extraneous variables (EV)
All variables that are not independent variables but could affect the results (DV) of the experiment. Extraneous variables should be controlled where possible.
Confounding variables
Variable(s) that have affected the results (DV), apart from the IV. A confounding variable could be an extraneous variable that has not been controlled.
Random Allocation
Randomly allocating participants to independent variable conditions means that all participants should have an equal chance of participating in each condition.
Order effects
Changes in participants’ performance due to their repeating the same or similar test more than once. Examples of order effects include:
(i) practice effect: an improvement in performance on a task due to repetition, for example, because of familiarity with the task;
(ii) fatigue effect: a decrease in performance of a task due to repetition, for example, because of boredom or tiredness.
What is the control in an experiment?
In an experiment , the control is a standard or baseline group not exposed to the experimental treatment or manipulation. It serves as a comparison group to the experimental group, which does receive the treatment or manipulation.
The control group helps to account for other variables that might influence the outcome, allowing researchers to attribute differences in results more confidently to the experimental treatment.
Establishing a cause-and-effect relationship between the manipulated variable (independent variable) and the outcome (dependent variable) is critical in establishing a cause-and-effect relationship between the manipulated variable.
What is the purpose of controlling the environment when testing a hypothesis?
Controlling the environment when testing a hypothesis aims to eliminate or minimize the influence of extraneous variables. These variables other than the independent variable might affect the dependent variable, potentially confounding the results.
By controlling the environment, researchers can ensure that any observed changes in the dependent variable are likely due to the manipulation of the independent variable, not other factors.
This enhances the experiment’s validity, allowing for more accurate conclusions about cause-and-effect relationships.
It also improves the experiment’s replicability, meaning other researchers can repeat the experiment under the same conditions to verify the results.
Why are hypotheses important to controlled experiments?
Hypotheses are crucial to controlled experiments because they provide a clear focus and direction for the research. A hypothesis is a testable prediction about the relationship between variables.
It guides the design of the experiment, including what variables to manipulate (independent variables) and what outcomes to measure (dependent variables).
The experiment is then conducted to test the validity of the hypothesis. If the results align with the hypothesis, they provide evidence supporting it.
The hypothesis may be revised or rejected if the results do not align. Thus, hypotheses are central to the scientific method, driving the iterative inquiry, experimentation, and knowledge advancement process.
What is the experimental method?
The experimental method is a systematic approach in scientific research where an independent variable is manipulated to observe its effect on a dependent variable, under controlled conditions.
Controlled Experiment – Definition, Process, Importance, Examples
What is Controlled Experiment?
Definition of controlled experiment, how does controlled experiment works, importance of controlled experiment, examples of controlled experiment, quiz practice, what is a controlled experiment, why are controlled experiments important, what is the difference between the control group and the experimental group, how is the independent variable different from the dependent variable, what are controlled variables, why is reproducibility crucial in controlled experiments, what is a confounding variable, can controlled experiments be conducted outside of a laboratory setting, how do researchers ensure that their results are statistically significant, what are the limitations of controlled experiments.
- A controlled experiment is a methodical scientific investigation in which a researcher deliberately manipulates a specific variable, termed the independent variable, to observe its impact on a system under study.
- The primary objective is to isolate the effects of this single variable by keeping other potential variables, known as controlled variables, constant. This ensures that any observed changes can be attributed solely to the manipulation of the independent variable.
- In the realm of biology, controlled experiments often necessitate creating a restricted environment for the organism under investigation. This restriction is crucial to mitigate the unpredictable influences of the natural environment and its myriad variables.
- Typically, in a controlled experiment, the subjects or entities being studied are categorized into two primary groups. The first group, known as the control group , is subjected to standard conditions without any alteration in the variable of interest.
- In contrast, the second group, termed the experimental group , undergoes a specific change in the variable being tested. The control group serves as a benchmark, facilitating a comparative analysis with the experimental group to discern any differences that arise due to the variable manipulation.
- Control mechanisms are integral to experimental design, ensuring the validity and reliability of the results. These controls act as reference points, confirming the efficacy of the experiment and providing a foundation for comparison.
- In the scientific community , for results to gain acceptance, they must demonstrate statistical significance. This means that the observed differences between the control and experimental groups should not be mere random occurrences. Statistical analyses assist in discerning whether the observed results substantiate the proposed hypothesis or if they might have occurred by mere coincidence.
A controlled experiment is a scientific investigation in which a researcher deliberately manipulates a specific variable, while keeping all other variables constant, to determine its effect on a system under study.
A controlled experiment works by systematically testing a hypothesis through the manipulation of one variable while keeping all other variables constant. Here’s a step-by-step breakdown of how it works:
- Hypothesis Formation: Begin by stating a clear hypothesis or prediction about the relationship between two variables.
- Independent Variable: This is the variable that the researcher will manipulate. It’s the presumed cause in the hypothesis.
- Dependent Variable: This is the variable that the researcher will measure to see if it changes in response to the independent variable. It’s the presumed effect.
- Controlled Variables: These are all other factors that could influence the outcome. They are kept constant throughout the experiment to ensure that any changes observed are solely due to the manipulation of the independent variable.
- Control Group: This group does not receive any treatment or manipulation of the independent variable. It serves as a baseline for comparison.
- Experimental Group: This group receives the treatment or manipulation of the independent variable.
- Conduct the Experiment: Apply the treatment to the experimental group while ensuring that the control group remains unaffected. Ensure that all other conditions remain the same for both groups.
- Data Collection: Measure and record the dependent variable for both the control and experimental groups.
- Analysis: Compare the results from the two groups to determine if there was a significant difference due to the manipulation of the independent variable.
- Conclusion: Based on the analysis, determine whether the hypothesis is supported or refuted. If the results are statistically significant, it suggests that the independent variable had an effect on the dependent variable.
- Replication: To validate the findings, the experiment should be repeatable by other researchers under the same conditions.
By following this systematic approach, controlled experiments aim to determine causal relationships between variables, eliminating potential confounding factors and ensuring that the observed effects are genuinely due to the variable being tested.
Controlled experiments are foundational to the scientific method and play a pivotal role in advancing knowledge across various fields. The importance of controlled experiments can be elucidated as follows:
- Causality Determination: Controlled experiments allow researchers to establish causal relationships between variables. By manipulating one variable (independent variable) and observing its effect on another (dependent variable), while keeping all other variables constant, researchers can ascertain if a change in one variable directly causes a change in another.
- Elimination of Confounding Variables: In a controlled experiment, all extraneous variables are kept constant, ensuring that any observed changes in the dependent variable are solely due to the manipulation of the independent variable. This minimizes the risk of external factors skewing the results.
- Reproducibility: The structured nature of controlled experiments ensures that they can be replicated by other researchers. Reproducibility is crucial for validating findings and ensuring that results are consistent across different settings and conditions.
- Objective Analysis: Controlled experiments provide a systematic and objective framework for testing hypotheses. This reduces biases and subjective influences, leading to more accurate and reliable results.
- Quantitative Data Collection: These experiments often yield quantitative data, which can be statistically analyzed to determine the significance of findings. This provides a robust basis for drawing conclusions and making informed decisions.
- Standardized Conditions: By maintaining consistent conditions across experimental and control groups, researchers can ensure that any observed differences are genuinely due to the variable being tested and not other extraneous factors.
- Facilitates Theory Development: Controlled experiments contribute to the development and refinement of theories. Positive results can support and strengthen existing theories, while unexpected results can lead to new hypotheses and avenues of exploration.
- Informs Policy and Practice: Findings from controlled experiments can have real-world implications, informing policy decisions, medical treatments, educational practices, and more. They provide evidence-based insights that can lead to better outcomes in various sectors.
- Ethical Considerations: In some cases, controlled experiments, especially in medical and psychological research, ensure that treatments or interventions are tested in a safe and ethical manner before being widely adopted.
- Enhanced Credibility: Results from well-designed controlled experiments are often viewed with higher credibility in the scientific community, as they adhere to rigorous standards of investigation.
In summary, controlled experiments are indispensable in the realm of scientific research, providing a rigorous and systematic approach to knowledge acquisition. They ensure that findings are valid, reliable, and grounded in evidence, paving the way for advancements in science and technology.
- Music Preference in Canines: In an intriguing exploration into the musical preferences of dogs, researchers sought to determine whether dogs exhibited differential behaviors in response to various music genres. The primary variable under investigation was the genre of music, designated as the independent variable. To ensure the validity of the results, several factors were meticulously controlled, including the ambiance of the room, music volume, human presence, and ambient temperature.The experimental setup was consistent, with identical lighting, furniture, and conditions for each test to eliminate any behavioral changes attributed to environmental factors. To further control external influences, the presence of humans was eliminated, and music was consistently played at a uniform volume across genres.For a comprehensive analysis, dogs were divided into two distinct groups. One group, termed the control group, was observed without any music to establish a baseline behavior. The other group, exposed to various music genres, was then compared to the control group. Behaviors were quantitatively assessed, and statistical methods were employed to discern significant behavioral differences. The culmination of this extensive research revealed a fascinating insight: dogs exhibited a marked preference for reggae music, demonstrating more relaxed and calm behaviors when exposed to this genre.
- Scurvy Amongst Sailors: The 18th century witnessed a surge in maritime exploration, with sailors embarking on long voyages. Their sustenance primarily comprised the most economical diets, which unfortunately lacked essential nutrients. This dietary insufficiency led to the onset of diseases like scurvy, a debilitating condition resulting from vitamin C deficiency. Early symptoms of scurvy are subtle, manifesting as fatigue. However, prolonged deficiency leads to the disintegration of blood vessels, culminating in internal bleeding and eventual death.Dr. James Lind of the Royal Navy, recognizing the severity of scurvy, initiated one of the earliest controlled experiments to identify an effective remedy. Sailors afflicted with scurvy were segregated into distinct groups, each receiving a specific treatment alongside their regular diet. While some groups were administered barley water or cider, one group received oranges and lemons. This pioneering clinical trial aimed to assess the efficacy of these treatments in a controlled environment.The results were groundbreaking. The group receiving oranges and lemons exhibited rapid recovery, highlighting the therapeutic potential of vitamin C. This discovery revolutionized naval dietary protocols, with the Royal Navy incorporating vitamin C-rich greens into sailors’ diets, effectively mitigating the prevalence of scurvy.
These examples underscore the significance of controlled experiments in scientific research, offering insights that have the potential to transform our understanding and address pressing challenges.
What is the primary purpose of a controlled experiment? a) To test multiple variables simultaneously b) To observe natural phenomena without interference c) To establish a causal relationship between variables d) To gather qualitative data on a subject’s feelings
In a controlled experiment, which variable is deliberately manipulated by the researcher? a) Dependent variable b) Controlled variable c) Independent variable d) Confounding variable
Which group in a controlled experiment does not receive the treatment or manipulation? a) Experimental group b) Control group c) Dependent group d) Independent group
What is the primary function of controlled variables in an experiment? a) To ensure the results are statistically significant b) To ensure that observed changes are due to the independent variable alone c) To add variability to the results d) To provide multiple outcomes for comparison
Why is reproducibility important in controlled experiments? a) To ensure the results are unique b) To validate the findings across different settings c) To increase the complexity of the experiment d) To reduce the sample size
Which of the following is NOT a characteristic of a controlled experiment? a) Systematic manipulation of variables b) Objective analysis of results c) Testing of multiple hypotheses simultaneously d) Keeping extraneous variables constant
In a controlled experiment, what does the dependent variable represent? a) The variable that is kept constant b) The variable that is manipulated c) The outcome or effect being measured d) The variable that adds randomness to the results
What role does the control group play in a controlled experiment? a) It undergoes the treatment being tested b) It serves as a benchmark for comparison c) It determines the independent variable d) It adds variability to the results
Which of the following best describes a confounding variable? a) A variable that is deliberately manipulated b) A variable that is kept constant throughout the experiment c) An external factor that affects the outcome and is not controlled d) The primary outcome measured in the experiment
Why are controlled experiments considered the gold standard in scientific research? a) They are the most expensive type of experiment b) They provide qualitative insights into human behavior c) They allow for the establishment of cause-and-effect relationships d) They are based on subjective observations
A controlled experiment is a scientific test where a researcher manipulates one variable and observes its effect on another, while keeping all other variables constant.
They allow researchers to establish causal relationships between variables, ensuring that observed changes are due to the manipulated variable alone.
The control group does not receive the treatment or manipulation, while the experimental group does. The control group serves as a benchmark for comparison.
The independent variable is the one that is deliberately manipulated by the researcher, while the dependent variable is the outcome or effect being measured.
Controlled variables are factors that are kept constant throughout the experiment to ensure that any observed changes are solely due to the independent variable.
Reproducibility ensures that results are consistent across different settings and conditions, validating the findings and enhancing their credibility.
A confounding variable is an external factor that can affect the outcome of an experiment but is not controlled by the researcher.
Yes, controlled experiments can be conducted in various settings, including field studies, as long as variables can be effectively controlled and manipulated.
Researchers use statistical analysis to determine if the differences observed between the control and experimental groups are significant and not due to chance.
Some limitations include potential ethical concerns, the challenge of controlling all variables in complex systems, and the possibility that results in controlled settings may not always generalize to real-world scenarios.
Related Biology Study Notes
Adaptation – definition, types, reasons, examples, non-communicable diseases – types, control, examples, communicable diseases – types, transmission, control, biological organization – history, levels, importance, diffusion – definition, causes, significance, examples, asexual reproduction – definition, types, advantages, examples, sexual reproduction – stages, types, advantages, examples, phenotype – definition, importance, examples, latest questions.
- All Questions
Start Asking Questions Cancel reply
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
- Click on your ad blocker icon in your browser's toolbar
- Select "Pause" or "Disable" for this website
- Refresh the page if it doesn't automatically reload

LEARN STATISTICS EASILY
Learn Data Analysis Now!

What is: Controlled Experiment
What is a controlled experiment.
A controlled experiment is a scientific test that aims to establish a cause-and-effect relationship between variables. In this type of experiment, researchers manipulate one variable, known as the independent variable, while keeping all other variables constant. This allows for a clear observation of how changes in the independent variable affect the dependent variable, which is the outcome being measured. Controlled experiments are fundamental in fields such as statistics, data analysis , and data science, as they provide reliable data that can be analyzed to draw valid conclusions.
Ad description. Lorem ipsum dolor sit amet, consectetur adipiscing elit.
The Importance of Control Groups
In a controlled experiment, the use of control groups is essential. A control group is a baseline group that does not receive the experimental treatment or intervention. By comparing the results of the experimental group, which receives the treatment, to the control group, researchers can determine the effect of the independent variable more accurately. This comparison helps to eliminate alternative explanations for the observed effects, thereby strengthening the validity of the experiment’s conclusions.
Randomization in Controlled Experiments
Randomization is a critical aspect of controlled experiments. It involves randomly assigning participants or subjects to either the experimental group or the control group. This process helps to ensure that any differences observed between the groups are due to the treatment rather than pre-existing differences among the participants. Randomization minimizes bias and enhances the reliability of the results, making it a cornerstone of rigorous experimental design in data science.
Types of Controlled Experiments
Controlled experiments can be categorized into different types, including laboratory experiments and field experiments. Laboratory experiments are conducted in a controlled environment where researchers can manipulate variables with precision . In contrast, field experiments take place in natural settings, allowing researchers to observe real-world behaviors while still maintaining control over certain variables. Each type has its advantages and disadvantages, and the choice depends on the research question and context.
Hypothesis Testing in Controlled Experiments
Hypothesis testing is a fundamental component of controlled experiments. Researchers formulate a hypothesis, which is a testable prediction about the relationship between the independent and dependent variables. The controlled experiment is designed to test this hypothesis, and statistical methods are employed to analyze the data collected. If the results support the hypothesis, it may be accepted; if not, it may be rejected or revised. This process is crucial for advancing knowledge in statistics and data analysis.
Data Collection and Analysis
Data collection in controlled experiments is systematic and structured. Researchers gather quantitative or qualitative data based on the outcomes they are measuring. This data is then analyzed using various statistical techniques to determine the significance of the results. The analysis helps researchers understand the impact of the independent variable on the dependent variable, providing insights that can inform future research and decision-making in data science.
Ethical Considerations in Controlled Experiments
Ethical considerations are paramount in conducting controlled experiments, especially those involving human subjects. Researchers must ensure that participants are fully informed about the nature of the experiment and provide their consent. Additionally, they must consider the potential risks and benefits of the study. Adhering to ethical guidelines not only protects participants but also enhances the credibility of the research findings in the field of data analysis.

Limitations of Controlled Experiments
While controlled experiments are powerful tools for establishing causality, they do have limitations. One significant limitation is the artificial nature of laboratory settings, which may not accurately reflect real-world conditions. Additionally, controlled experiments may not always be feasible or ethical in certain situations, such as when studying complex social behaviors. Researchers must be aware of these limitations and consider complementary research methods to gain a comprehensive understanding of the phenomena being studied.
Applications of Controlled Experiments
Controlled experiments have wide-ranging applications across various fields, including psychology, medicine, and marketing. In psychology, they are used to study behavioral responses to different stimuli. In medicine, controlled clinical trials are essential for testing the efficacy of new treatments. In marketing, controlled experiments help businesses understand consumer preferences and optimize their strategies. The versatility of controlled experiments makes them invaluable in generating actionable insights from data analysis.
Biology Simple
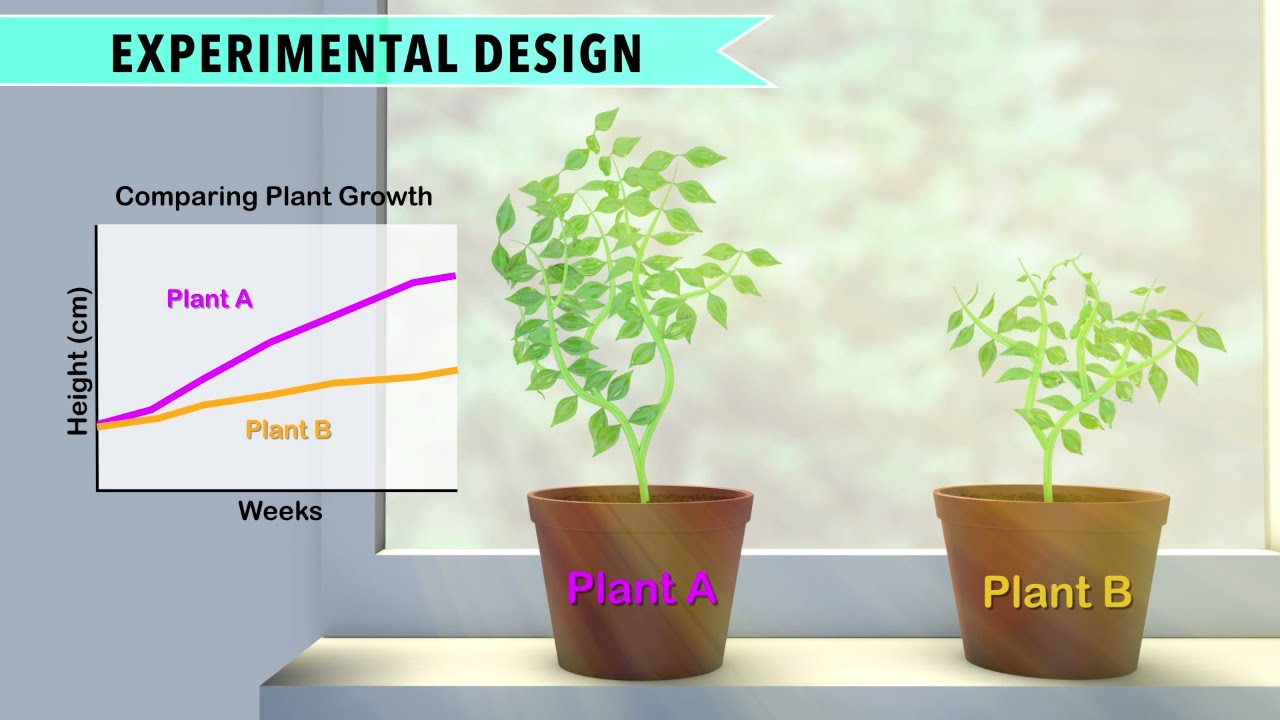
Controlled Experiment
A controlled experiment is a scientific study where variables are carefully manipulated and controlled. It helps researchers establish cause-effect relationships.
In the realm of scientific research, controlled experiments hold significant importance for exploring and understanding various phenomena. By systematically adjusting and regulating specific variables, researchers can draw accurate conclusions and establish causal relationships. This methodical approach allows for the isolation of key factors influencing the outcomes, leading to reliable and reproducible results.
Through controlled experiments, scientists can unravel complex patterns, test hypotheses, and make informed decisions based on empirical evidence. In essence, controlled experiments serve as a cornerstone in the scientific method, providing a structured framework for inquiry and discovery.

Credit: www.simplypsychology.org
Designing A Controlled Experiment
In the process of conducting a controlled experiment, designing plays a pivotal role in setting the stage for accurate and reliable results. Each step of the design phase requires careful thought and attention to detail, as it ultimately dictates the validity of the entire experiment. From identifying the research question to formulating hypotheses and selecting variables, every aspect of the experiment’s design demands thorough consideration.
Identifying The Research Question
The first step in designing a controlled experiment is identifying the research question. This question serves as the foundation upon which the entire experiment is built. Ensuring that the research question is clear, specific, and measurable is essential to establishing a solid framework for the experiment.
Formulating The Hypothesis
After identifying the research question, the next step involves formulating the hypothesis. The hypothesis should clearly outline the relationship between the variables being studied and is vital in guiding the direction of the experiment.
Selecting The Variables
Upon formulating the hypothesis, selecting the variables to be studied is crucial. This process involves identifying and defining the independent and dependent variables, as well as any extraneous variables that could potentially impact the results.
Developing The Control Group
Another integral component of designing a controlled experiment is developing the control group. The control group serves as the baseline for comparison and allows researchers to isolate the effects of the independent variable on the dependent variable.
Randomization And Sample Size
Randomization and determining the appropriate sample size are critical aspects of experimental design. Random assignment helps minimize the influence of confounding variables, while a sufficient sample size ensures that the results are representative of the population being studied.
Conducting A Controlled Experiment
- Define clear objectives and hypotheses for the experiment.
- Create a detailed experimental plan with specific steps.
- Apply the treatment to the experimental group as planned.
- Ensure the control group receives no treatment for comparison.
- Use reliable tools and methods to collect data accurately.
- Record all data points meticulously for analysis.
- Regularly check and adjust factors that could impact the experiment.
- Keep conditions consistent across all groups throughout the experiment.
- Document all observations in a structured format for analysis.
- Ensure all researchers adhere to the observation recording procedure.
Analyzing And Interpreting Results
This study examines the controlled experiment on analyzing and interpreting results, providing valuable insights into the research process to drive decision-making. Discover how data analysis and interpretation play a crucial role in drawing meaningful conclusions and optimizing outcomes.
Data Analysis Techniques
In a controlled experiment, data analysis techniques play a crucial role.
Identifying Trends And Patterns
Identifying trends and patterns helps uncover valuable insights.
Drawing Conclusions
Drawing conclusions from the data leads to actionable outcomes.
Evaluating The Validity And Reliability
Ensuring the validity and reliability of results is essential.
Validation reinforces the credibility of the experiment.
Reliability ensures consistent and trustworthy outcomes.
Thorough analysis aids in assessing the experiment’s success.
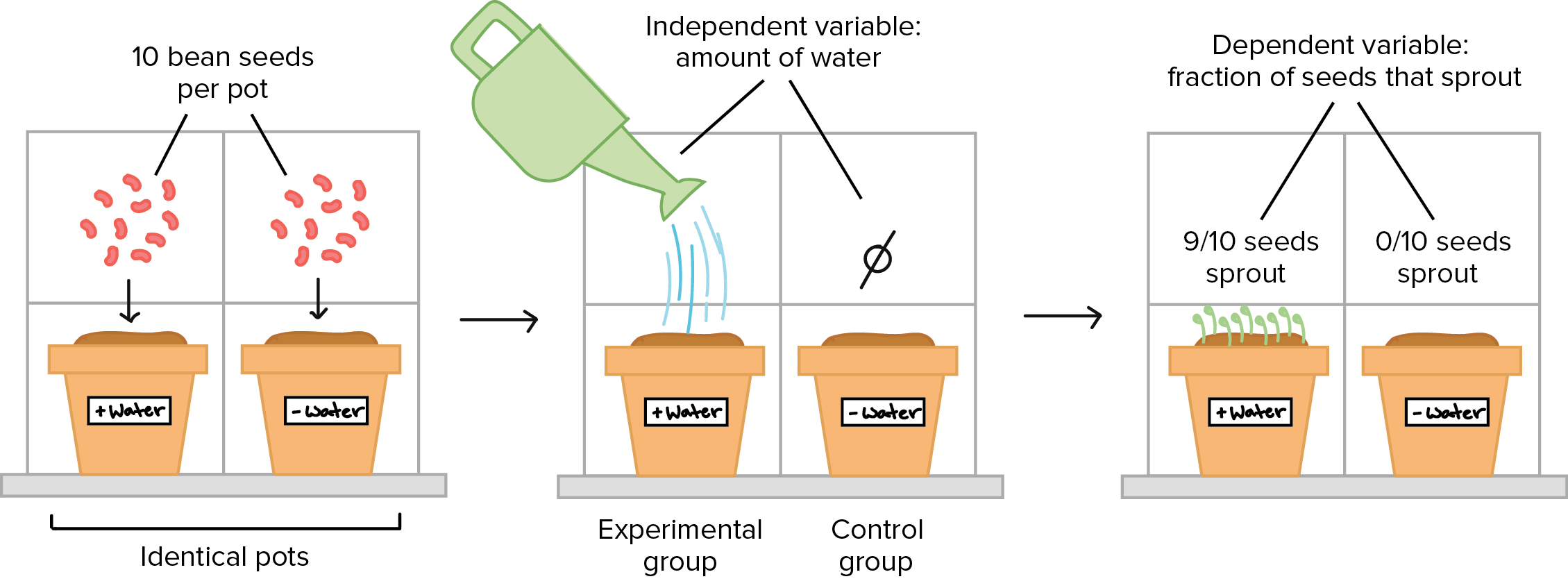
Credit: www.khanacademy.org
Limitations And Considerations
In any controlled experiment, it is crucial to consider the limitations and various factors that may impact the study’s outcomes. Understanding potential biases, ethical considerations, generalizability and external validity, limitations of control groups, and addressing confounding variables are essential in ensuring the reliability and validity of the results.
Potential Biases
Potential biases, such as selection bias, measurement bias, or observer bias, can significantly affect the results of a controlled experiment. It’s important to identify and mitigate these biases to ensure the accuracy of the findings.
Ethical Considerations
Ethical considerations play a vital role in the planning and execution of a controlled experiment. It’s essential to uphold ethical standards, including informed consent, safeguarding participants’ privacy, and minimizing any potential harm or distress.
Generalizability And External Validity
Generalizability and external validity refer to the extent to which the findings of a controlled experiment can be applied to a broader population or real-world settings. It’s important to consider these factors to determine the practical implications of the study.
Limitations Of Control Groups
The limitations of control groups, such as ensuring they accurately represent the population being studied and minimizing the impact of variables, need to be carefully addressed to enhance the credibility of the experiment.
Addressing Confounding Variables
Identifying and addressing confounding variables is crucial in controlling for extraneous factors that could influence the outcomes of the experiment. Proper techniques, such as randomization and statistical controls, should be employed to minimize the impact of confounding variables.

Credit: explorebiology.org
Frequently Asked Questions Of Controlled Experiment
What is the meaning of controlled experiment.
A controlled experiment is a research method where variables are carefully controlled to measure the effects of one variable on another. It allows researchers to establish cause and effect relationships by eliminating confounding factors.
What Is An Example Of A Controlled Study?
An example of a controlled study is a clinical trial where participants are assigned to different groups, one receiving the treatment and the other a placebo. This helps to measure the effectiveness of the treatment while controlling for other variables.
What Is The Difference Between Controlled And Uncontrolled Experiments?
Controlled experiments involve manipulating variables, while uncontrolled experiments do not. Controlled experiments offer more reliable results due to the controlled conditions.
What Is A Controlled Cause To Effect Experiment?
In a controlled cause to effect experiment, variables are carefully manipulated to observe specific outcomes.
What Is A Controlled Experiment?
A controlled experiment is a scientific study where variables are carefully controlled to determine cause and effect.
To sum up, conducting controlled experiments is crucial for obtaining accurate and reliable results. By carefully controlling variables, researchers can better understand cause-and-effect relationships. This method enhances the credibility and applicability of scientific findings. As a result, controlled experiments play a vital role in advancing knowledge and innovation across various fields.
Similar Posts
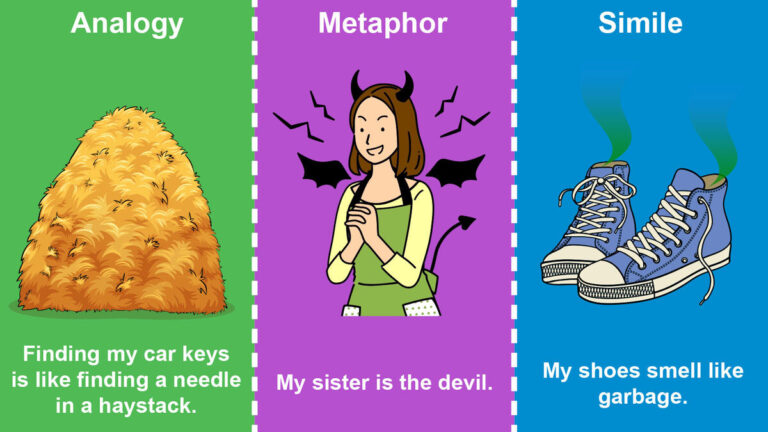
An analogy is a comparison that helps explain a concept by drawing similarities between two different things. Introducing analogies can be an effective way to clarify complex ideas and make them more understandable. By drawing parallels between two distinct concepts, analogies enable readers to grasp unfamiliar concepts by relating them to something more familiar. This…
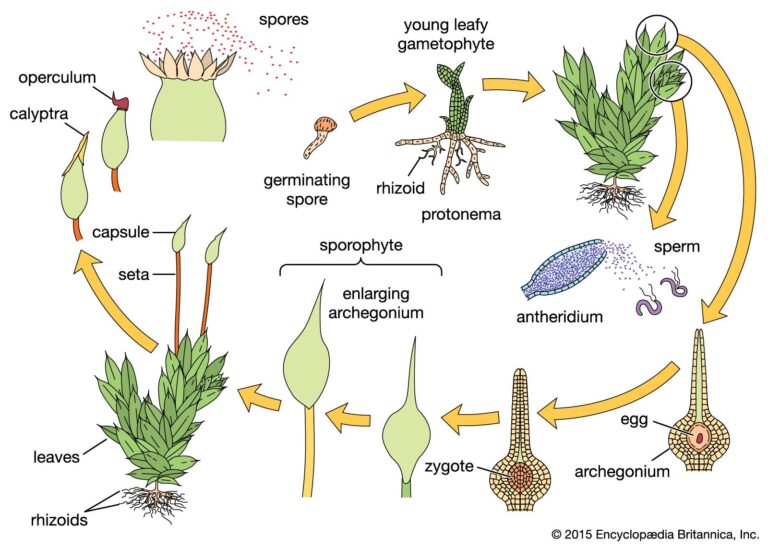
Bryophytes are non-vascular land plants that include mosses, liverworts, and hornworts and are an important component of damp habitats. These plants have a thallus-like body attached to the substrate by rhizoids and lack true vegetative structures. They reproduce through gametes and have a haploid main plant body called a gametophyte. Dioecious bryophytes have separate male…
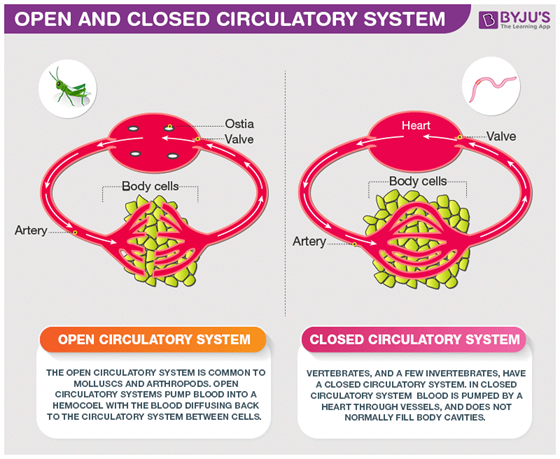
Closed Circulatory System
A closed circulatory system is a type of circulatory system where blood flows within vessels. This system is more efficient in transporting nutrients and oxygen throughout the body by using a network of blood vessels. It enables the blood to circulate under higher pressure, which enhances the transport of essential substances to the cells and…
Atom is a text editor developed by GitHub, known for its simplicity and flexibility for coding. It is widely used by developers for writing and editing code efficiently. With a clean interface and customizable features, Atom supports various programming languages and offers a range of packages and themes to enhance the coding experience. Atom’s collaborative…
A flagellum is a whip-like appendage that allows microorganisms to move and swim. It is a long, slender structure made of protein and is found in bacteria, archaea, and eukaryotic cells. Flagella play a crucial role in the mobility and survival of many single-celled organisms. They enable microorganisms to navigate their environment, seek nutrients, and…
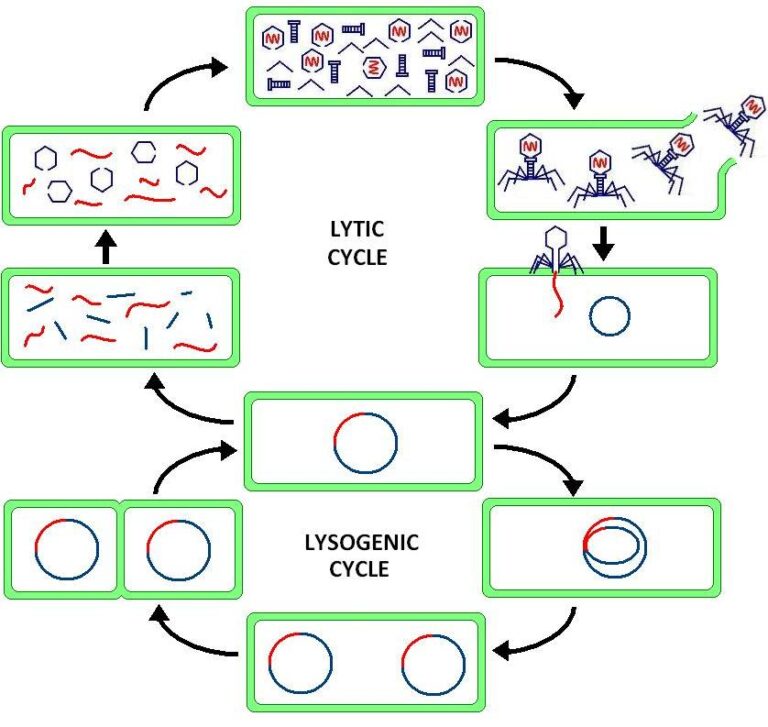
Lysogenic Conversion
Lysogenic conversion refers to the process where a bacteriophage integrates its DNA into the host bacterium’s genome. This integration can lead to the expression of new traits in the host bacteria. Lysogenic conversion is a fascinating phenomenon in microbiology, where a bacteriophage, or virus that infects bacteria, can influence the genetic makeup of its host….
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

IMAGES
VIDEO
COMMENTS
A hypothesis is a testable prediction about the relationship between variables. It guides the design of the experiment, including what variables to manipulate (independent variables) and what outcomes to measure (dependent variables). The experiment is then conducted to test the validity of the hypothesis.
The Controlled Experiment, Hypothesis Testing, and the z Distribution 5 Chapter 5 Goals ... on the dependent variable compared to the control group. Hypothesis Testing: The Big Decision_____ All experiments begin with the statement of the null and alternative hypothe- ses (at least in the experimenter's mind, but not usually in the published ...
In a controlled experiment, ... Experimental design is the process of planning an experiment to test a hypothesis. The choices you make affect the validity of your results. 1520. How to write a lab report A lab report conveys the aim, methods, results, and conclusions of a scientific experiment. 603.
For example, a controlled experiment examined whether a new teaching method improves test scores. Researchers randomly assigned students to either the new or traditional method groups. This random assignment ensured that factors like prior knowledge, motivation, and ability were equally distributed.
A controlled experiment works by systematically testing a hypothesis through the manipulation of one variable while keeping all other variables constant. Here's a step-by-step breakdown of how it works: Hypothesis Formation: Begin by stating a clear hypothesis or prediction about the relationship between two variables.
A controlled experiment is a scientific test that is directly manipulated by a scientist, in order to test a single variable at a time. ... can use the difference between the control group and experimental group and the expected difference to determine if the experiment supports the hypothesis, or if the data was simply created by chance ...
To reach effective results, you need to test your hypothesis by performing an experiment, but it's not as if any random experiment can give you results. ... A controlled experiment basically limits the scope of the result because only one or two factors affecting the result are allowed to vary. All the other factors are kept constant.
Hypothesis Testing in Controlled Experiments. Hypothesis testing is a fundamental component of controlled experiments. Researchers formulate a hypothesis, which is a testable prediction about the relationship between the independent and dependent variables. The controlled experiment is designed to test this hypothesis, and statistical methods ...
General: Hypothesis testing Hypothesis = prediction of the outcome of an experiment. Framed in terms of independent and dependent variables: A variation in the independent variable will cause a difference in the dependent variable Aim of the experiment: prove this prediction By disproving the "null hypothesis"
A controlled experiment is a scientific study where variables are carefully manipulated and controlled. ... Through controlled experiments, scientists can unravel complex patterns, test hypotheses, and make informed decisions based on empirical evidence. ... the next step involves formulating the hypothesis. The hypothesis should clearly ...