Stack Exchange Network
Stack Exchange network consists of 183 Q&A communities including Stack Overflow , the largest, most trusted online community for developers to learn, share their knowledge, and build their careers.
Q&A for work
Connect and share knowledge within a single location that is structured and easy to search.

Methods to stabilize and maintain extremely low humidity in a lab environment
My atomic physics lab is in a building that experiences huge swings in humidity levels during the year due to the monsoon season
Our building provides temperature, but not humidity control. Using just the building temperature control results in the following lab climates:
10 months out of the year, the room is at
T $\approx 24.4 ^oC$
Relative Humidity $< 10\%$
2 months out of the year, the lab is at
T $\approx 24.4^oC$
Relative Humidity $\approx 50\%$
This season variation necessitates significant recalibration twice per year at the beginning and end of the monsoon season. The sensitive components are mainly opto-mechanical.
The lab currently has a dehumidifier that is spec'd at 45 pints per day during the wet season. This specification indicates how much water the unit will remove from the air in a given day when the air is saturated with water (100% relative humidity). The problem with such a specification is that 100% relative humidity is a way different environment than 40% or 50% humidity.
On a wet day, this unit reduces the lab relative humidity by about 10% from 55% to 45%. This is still far from the lab's climate most of the year. It is a trade off, though, because it will also raise the lab temperature by about 1 degree C, which necessitates other recalibration. I am investigating options to further reduce the humidity.
The lab is approximately 5 meters X 10 meters X 3 meters in size. Most of the experiment is on a very full optics table that is 1.5 meters X 4 meters. There are lots of cables and water tubing that require access to the table, making climate isolation of the table difficult (although not impossible).
A few options under consideration are the following:
1: Introduce an additional higher capacity dehumidifier
- Fast and easy implementation
it is unknown how efficient a dehumidifier will function when the relative humidity is only 45%.
Manufacturers do not specify how well the unit will work at low humidity levels, only at 80% +.
2: Fill sensitive areas with positive pressure Nitrogen
- Excellent climate control
- Minimal impact on room temperature
- requires significant reconfiguration of experimental setup.
- Requires refilling Nitrogen tank frequently, a recurring cost.
3: Isolate experiment from lab climate using large plastic enclosures and recirculate air in this enclosure
- Excellent environment isolation
- requires significant reconfiguration of laboratory and would likely restrict access to areas of the experiment.
- It could also likely result in a temperature increase of the experiment area.
Introducing an additional room dehumidifier would be the easiest option by far.
So my Question is: does anyone know how efficient dehumidifiers works in dry environments? E.g., if I were to purchase an additional dehumidifier, would could I achieve a humidity level of less than 30% or does the humidity level asymptote off at some level due to a limit on the efficiency of dehumidifiers?
I realize an alternative would be to humidify the lab 10 months out of the year. However, having low humidity is extremely convenient for rapidly water-cooling components. During our wet season, our water-cooling results in considerable condensation on our components.
- experimental-physics
- experimental-technique
- experimental-technology
- climate-science
- 1 $\begingroup$ Chalk up to the list of Nitrogen's cons: danger to life and limb in case of a leak. $\endgroup$ – Deer Hunter Commented Jun 27, 2013 at 20:41
- $\begingroup$ Joe - check out Chapter 23 of ASHRAE's HVAC Systems and Equipment Handbook (2008). $\endgroup$ – Deer Hunter Commented Jun 27, 2013 at 20:46
- 2 $\begingroup$ I found an interesting external link: Desiccant Dehumidification vs. Mechanical Refrigeration ( bry-air.com/… ) In summary, their recommendation is that desiccant based dehumidifiers are necessary if you need relative humidity levels between 1% and 45% $\endgroup$ – Joe Commented Jun 27, 2013 at 22:20
- $\begingroup$ Maybe you could use slightly warmer cooling water to prevent condensation? Are you using open-cycle cooling - slightly wasteful. $\endgroup$ – akrasia Commented Aug 14, 2014 at 13:18
2 Answers 2
One option: Buy more dehumidifiers, and a space heater. Use the space heater to maintain the lab at the "elevated" temperature when the humidity is low and the dehumidifiers are not running.
Another option: Run your dehumidifier when the humidity is high, but run a humidifier when the humidity is low. Meet in the middle.
Yet another option: Build a tent around your optics table and control the climate in there. Less air volume means less challenge. The tent probably doesn't have to be completely airtight.
It is probably not an answer good enough, but I try. My experiences with a small commercial dehumidifier were great when humidity was 100%. I tested it long term at 60% without big effect.
I was trying to decrease humidity with Calcium Chloride type material and ventilator (Silica gel is probably safer but less efficient), it worked better but 10%, I have never seen. But maybe with a lot of material... Actually I am impressed that you have 10%, even your presence must influence it...
Your Answer
Sign up or log in, post as a guest.
Required, but never shown
By clicking “Post Your Answer”, you agree to our terms of service and acknowledge you have read our privacy policy .
Not the answer you're looking for? Browse other questions tagged experimental-physics experimental-technique experimental-technology climate-science or ask your own question .
- Featured on Meta
- Preventing unauthorized automated access to the network
- Upcoming initiatives on Stack Overflow and across the Stack Exchange network...
- Our Help center policy article on generative AI content
Hot Network Questions
- Rationale for requiring struct prefix in C
- Shading problem with Game Asset
- Stick lodging into front wheel - is it preventable?
- How to fix frayed Magsafe 3 cable?
- What's a realistic cadence for refueling an HLS Starship on-orbit?
- Opening URL in the Windows default Browser using C
- How should I handle students who are very disengaged in class?
- How can bounded orbits diverge arbitrarily far at late times?
- Finding the MLE using a contour plot
- How to prevent partition-only differences in QAT output?
- "Riiiight," he said. What synonym of said can be used here?
- Hyperbolic manifolds and their fundamental group
- Stationary phase formula for a complex valued phase
- Customize Sitecore crawling logs to log request payload and request url on rebuild index
- How to mitigate fading of SW reception
- SSL certificate working ok on Firefox but not for Chrome
- How to typeset such table?
- Newbie with a grease gun - lacking a bleeder valve
- Nexus hub - why is the sprocket being dragged round by a dust cap?
- What is this 4 element monoid?
- How good is Quicken Spell as a feat?
- Is observation the only way to indicate that God is real?
- Your Average Character
- How much knowledge and effort is required to see Northern Lights?

Humidity in materials research, including solar cell research
Humidity: A Vital but Often Overlooked Parameter in Materials Research
Temperature and relative humidity must be carefully controlled in numerous fields of research.
View Full Profile .
Learn about our Editorial Policies .
Humidity can significantly impact the properties of a material, such as its cosmetic surface, and mechanical and chemical properties. Temperature and relative humidity (RH) must be carefully controlled in numerous fields of research, from designing energy storage systems to developing stable pharmaceutical ingredients. Although the two are intrinsically linked, temperature is often given greater consideration in experimental design than humidity, which is more challenging to control with high accuracy and reproducibility.
Strategies for controlling humidity vary, with many researchers using a chamber containing salts (e.g. NaCl, RH 75%) to achieve constant RH. However, these methods are not designed to provide controlled humidity variation and as such, designing experiments with RH as a controlled variable is challenging. Dedicated humidity control systems are increasingly used with analytical methods such as light microscopy, Raman, Fourier transform infrared (FT-IR) spectroscopy, and X-ray to characterize how materials behave in different environmental conditions. Advanced systems are now able to control humidity across 5%–90% RH at a range of temperatures, allowing researchers to precisely control water vapor in the environment surrounding the sample for long periods of time.
Get training in Asset Management and earn CEUs .
One of over 25 IACET-accredited courses in the Academy.
Asset Management course
Here, we demonstrate the importance of controlling humidity in materials research, outlining three examples where a modern humidity controller has been successfully used to further understanding of how materials behave to develop optimized materials for a range of purposes.
Cure kinetics of silicone elastomers

Silicone sealants are used in several industrial, commercial, and domestic applications and are favored for their low modulus, electrical insulation, hydrophobicity, and low toxicity. Room-temperature vulcanizing (RTV) compounds are commonly used in silicone sealants which, once dispensed from an applicator, undergo a curing process and can be manipulated in situ until the cure is complete and the sealant is fully adhered to the substrate.

Most silicone RTV sealants rely on chemical reactions with water from the atmosphere to initiate and complete the curing process, and their cure kinetics under different environmental conditions have been the subject of research interest over many years. Studies into these so-called moisture-scavenging polymers have shown that temperature and RH have a significant impact on cure rates. 1,2,3
Researchers at the University of Nottingham recently investigated the combined roles of temperature and humidity on the cure of an RTV silicone adhesive putty, known commercially as Sugru ® and as Formerol ® F10 (FormFormForm Ltd., UK) in industrial markets. 4 Unlike many RTVs, this adhesive takes the form of a ductile solid in its pre-cured state, rather than a viscous fluid, making in situ shaping easier. It cures via condensation and hydrolysis reactions, where the initiation of cross-linking occurs upon exposure to atmospheric moisture.
The group measured the polymer’s cure progression via the shear modulus (measure of the elastic shear stiffness) as a function of time under controlled temperature and humidity conditions, to generate predictive models for cure timescales in relation to these environmental conditions. Conditions were controlled using a rheometer fitted with a sealed CTD450 environmental chamber fed by a humidity controller (Figure 1).
Interested in Analytical News?
By subscribing, you agree to receive email related to Lab Manager content and products. You may unsubscribe at any time.

By coupling a humidity controller to a rheometer, characteristic cure timescales for a commercial and industrial silicone elastomer could be established under controlled environmental conditions. Results showed that, at constant absolute humidity, an increase in temperature from 19°C to 39°C reduced the curing timescale by approximately half (Figure 2). An increase in humidity from 7.6% to 36.7% RH (at constant temperature) reduced the cure timescale from approximately 11 hours to four hours (Figure 3). Understanding the cure behavior of such materials not only gives an indication of how fast the silicone will cure in different parts of the world where climates differ substantially, but can also be used to speed up or slow down cure rates where necessary (to increase molding time or solidify quicker). This is particularly important in a production environment where time savings translate into cost savings.
Energy storage properties of thermochemical materials
The technology that enables homes and businesses to run off renewable energy has advanced enormously in recent years to meet a growing demand for this energy source. To successfully harness solar energy, however, energy storage systems must be able to bridge the disconnect between supply and demand. Salt hydrates are a class of thermochemical material used to develop heat batteries that can supply low-temperature thermal energy in residential buildings during colder periods. Their high density and ability to store energy without significant losses make salt hydrates a popular material for heat batteries.
A reversible chemical reaction between the salt hydrate and water vapor governs this principle of energy storage, where the solid salt M combines with water vapor to initiate energy discharge:
M • a H 2 O(s) + ( b - a ) H 2 O(g) ⇌ M • b H 2 O(s) + Energy
The hydrated salt can be recharged by applying heat energy to the system, which converts the salt back to a lesser hydrated state and releases water vapor. Water vapor and temperature govern whether a salt is in a hydrated or dehydrated (anhydrous) state, and the hydration rate is defined as the extent of the transformation of a lower hydrated or dry salt into a higher hydrated state per unit of time. Repeatedly hydrating and dehydrating a salt is known as “cycling,” and there is significant research interest in the effects of repeated cycling on salt hydrate morphology and hydration rate.
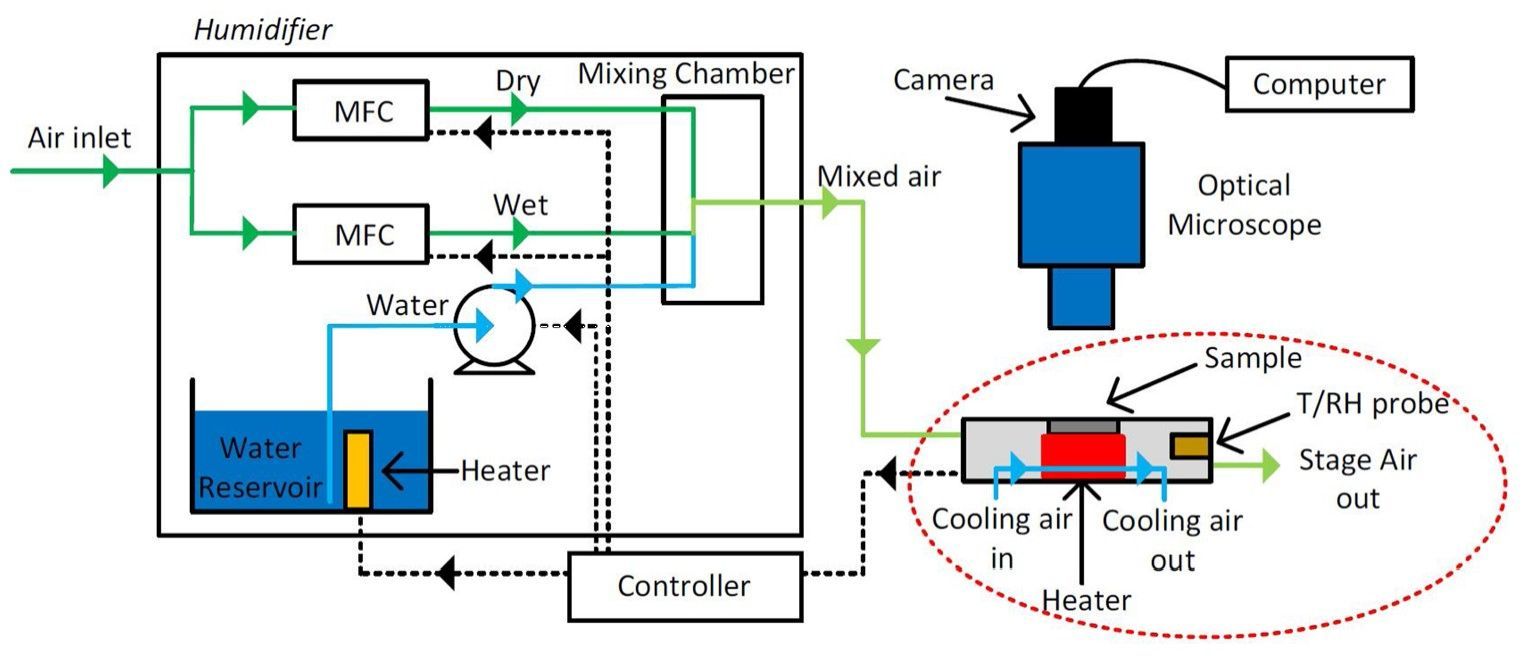
A group at the Eindhoven University of Technology investigated changes in particle size and crack formation of potassium carbonate (K 2 CO 3 ) particles in a microclimate chamber using optical microscopy. 5 The researchers hypothesized that crack formation over repeated cycles increases the hydration rate of the salt hydrate, as the expansion and breaking of the particle increases its microporosity and promotes water transport.
The microclimate chamber, also known as a “hot-stage” was connected to a humidity controller which enabled precise control over the experimental temperature and RH (Figure 4). The hydration rate of the K 2 CO 3 particles was evaluated by thermo gravimetric analysis (TGA), through simultaneous thermal analysis and the group developed a nucleation and growth model to describe the salt’s solid-state reactions, which incorporated crack formation.

K 2 CO 3 particles underwent 12 cycles in the hot-stage and their size was measured after each dehydration step, with an image being captured every 10 minutes by the optical microscope to show the “apparent area” (Figure 5). The particles were found to increase in size by roughly 30 percent over the 12 cycles. This increase was attributed to the cracking of individual crystals, confirming the hypothesis that cycling K 2 CO 3 particles increases their size through crack formation. TGA experiments revealed that cycling K 2 CO 3 particles significantly reduces the hydration rate, from approximately 8.3 hours for the first cycle to 33 minutes for the 12 th cycle. Together with the humidity- and temperature-controlled microscopy experiments, these results confirm the observation that an increase in salt hydrate particle size leads to a faster rate of hydration.
Extending the lifetime of solar cells
The effect of temperature and humidity on another renewable energy technology—solar cells, or photovoltaics (PVs)—is gaining increasing attention in the materials research community. Perovskite PVs have undergone rapid improvements in recent years and demonstrated considerable power conversion efficiency. Perovskite PVs have a classic layered structure composed of a transparent conducting oxide, a hole transport layer, a perovskite active layer, an electron transport layer, and an electrode such as gold (Au) or aluminum (Al).
However, their stability and durability represent a significant challenge in developing these devices for commercialization. According to the International Electrotechnical Commission's (IEC) standards, solar cells must perform well under non-laboratory conditions, such as in damp conditions (i.e. 85% humidity at 85°C) for more than 1,000 hours consistently. 6 Precise control of temperature and humidity in perovskite PV research and development that takes into account the multiple layered structure is therefore critical.
One study used temperature and humidity control devices with in situ Raman spectroscopy to investigate perovskite PV degradation mechanisms, to better understand the role of these environmental conditions on degradation kinetics. 7 Raman spectroscopy also allows researchers to examine the degradation of individual perovskite layers. Results from the in situ Raman humidity experiments showed that the dihydration of perovskite is almost completely reversible once drying occurs (Figure 6). Deeper analysis revealed that dihydration remained at the Au region, indicating that some moisture remained trapped in this region. It was deduced that PV device performance could be fully recovered if the trapped moisture could be removed.
Increasing the bond strength between the organic component of perovskite PVs and the metal halides could reduce the effect of potentially irreversible damage to the device due to trapped moisture, 8 and hydrophobic interlayers can also be introduced to help protect the perovskite from ambient moisture. 9 Experiments that can control humidity and measure its impact on different layers within perovskite PVs will be vital in the efforts to develop this type of solar cell for market.
Future of humidity control
The examples presented in this article provide a small snapshot of the huge number of applications benefiting from humidity and temperature control devices. These devices, which can be paired with a wide range of analytical methods, help researchers gain a deeper understanding of how materials behave under various environmental conditions. Recent advances in humidity control technology offer superior sensitivity and precision to accurately replicate the environmental conditions that a material could be subject to.
References:
- Halasz L and Belina K, An investigation into the curing of epoxy powder coating systems, J. Therm. Anal. Calorim . 119 (2015) 1971–1980.
- Hong I and Lee S, Cure kinetics and modeling the reaction of silicone rubber, J. Ind. Eng. Chem . 19 (2013) 42–47.
- Comyn J, Moisture cure of adhesives and sealants, Int. J. Adhesion Adhes . 18 (1998) 247–253.
- Elsmore MT and De Focatiis DSA, Combined roles of temperature and humidity on cure of a silicone elastomer, Polymer Testing , 93 (2021) 106967.
- Beving MAJM, Frijns AJH, Rindt CCM, and Smeulders DMJ, Effect of cycle-induced crack formation on the hydration behaviour of K 2 CO 3 particles: Experiments and modelling. Thermochimica Acta , 692 (2020) 178752.
- International Standard IEC61215. Terrestrial Photovoltaic (PV) Modules—Design Qualification and Type Approval—Part 1: Test Requirements and—Part 2: Test Procedures, 1.0 ed.; TC 82—Solar Photovoltaic Energy Systems; IEC: Geneva, Switzerland, 9 March 2016.
- Hooper KEA, Lee HKH, Newman ML, et al. Probing the degradation and homogeneity of embedded perovskite semiconducting layers in photovoltaic devices by Raman spectroscopy, Phys. Chem. Chem. Phys ., (2017) 19, 5246.
- O’Kane M, Perovskite Solar Cells: Causes of Degradation, Ossila Ltd. https://www.ossila.com/pages/perovskite-solar-cell-degradation-causes [Accessed 16/08/2021]
- Rajagopal A, Yao K, and Jen AKY, Towards Perovskite Solar Cell Commercialization: A Perspective and Research Roadmap Based on Interfacial Engineering, Advanced Materials (2018) 30(32): 1800455.
About the Author
Robert gurney, linkam scientific instruments, related topics.
- FTIR / NIR ,
- Materials Science ,
- Microscopy ,
- Product Resource: Insights ,
- Separations & Analysis
CURRENT ISSUE - October 2024

Lab Rats to Lab Tech: The Evolution of Research Models
Ethical and innovative scientific alternatives to animal-based R&D

Industry Expertise Improves Capsid Separation

Overcoming Contamination and Environmental Instability with CO2 Incubators

How to Cut Costs without Cutting Staff

Thermo Scientific™ General Purpose X Pro Centrifuges—Powerful at Every Turn

Stay Connected with Analytical News
Click below to subscribe to Analytical Tools & Techniques eNewsletter!
- United States
- United Kingdom
- Switzerland

March 5, 2020
Maintaining Laboratory Temperature and Humidity
- Humidity Monitoring
- Laboratory Monitoring
- Temperature Monitoring
When doing precision work, any alteration to the environment, the equipment, or the samples can have cascading effects. Even seasoned veterans run into issues in explaining if their results are possibly contaminated .
To do the best laboratory work, one needs to be able to reasonably rely on their results. These are some of the reasons that controlling laboratory temperature and humidity are so important. Of course, understanding this importance is only one piece of the pie.
Achieving a consistent set of conditions requires a lot of time and effort. So much that it leaves less time for the work. To get ahead of the risk factors, acquiring proper monitoring equipment and knowing tolerances is key.
Enjoy this deeper dive into information previously covered here .
Laboratory Temperature Guidelines
Every nation and organization has its own tweaks to the set standards for laboratory regulation. The agreed-upon standards set out by the International Standards Organisation (ISO) often form a baseline suggestion, rather than the actual hard and fast rule.
As an example, the optimal temperature listed is 20 -25 degrees celsius. This window is both overly broad and way too tight, depending on the work being done.
These temperatures are also for the samples themselves, not the ambient conditions. Measuring the temperature at the test site is difficult.
Maintaining
Testing the temperature at a sample provides a snapshot of the temperature range that is experienced. A sample goes through numerous changes. Movement from storage to a microscope element to a centrifuge all apply energy that changes temperature.
For the energy to raise the temperature outside of the window it needs to cross a threshold. Still, it is difficult to know if it has ever crept too high or too low.
And that kind of doubt can toss a breakthrough into the waste bin.
To this end, temperature monitoring needs to be done at each point in the process and listed before and after each test. This gives a more total range. The downside you face is the increased time to perform these tests. Time that leaves the sample exposed to other elements.
You can’t be aware of the temperature at all times, so you need to choose what points to collect and rely on a system that produces consistent results.
Monitoring each piece of equipment in a lab manually is both not ideal and practically impossible. Computer-assisted monitoring and external data collection free up value research man-hours. They also provide a more accurate data set.
Automatically adjusting heating and air conditioning systems is vital, especially for labs that have a fluctuating number of employees. The presence of additional personnel is one of the most easily overlooked environmental changes.
Laboratory Humidity Guidelines
Guidelines for humidity control recommend levels stay between 30 to 50 percent. Again, like the temperature guidelines, these are baselines set out by the ISO that see heavy modification based on need.
The relative humidity is somewhat easier to control than temperature for a sample. The particulars of the ambient humidity still face changes from one lab area to another.
Testing should be done within the range of typical storage and sample analysis areas to create a data set of variance. Length of exposure to humidity also needs to be taken into account.
Air purity is another important factor, so researchers should work with masks when near samples. This limits breath contamination. Alternatively, a positive pressure should be maintained to keep air quality in range.
Humidity monitors should be placed more centrally to equipment. Placing them near doors or portals where frequent changes happen can skew data points.
Contamination
The primary reason to avoid changes in temps/humidity is to avoid contamination. The secondary reason is to maintain sample integrity.
Contamination and degradation happen in varying degrees. Some of it affects the sample itself while others affect the transportation and storage media used.
Samples risk contamination from microbial growth both internal and externa l. A bioactive substance needs to be monitored for activity to know what stage of growth it experiences or if stasis has failed.
When a sample drifts out of the expected range, the growth of additional elements may ruin the sample outright. This is a secondary concern to the loss of containment. It’s difficult to accurately record growth rates if variables get out of hand or are unknown.
Samples also face infiltration from transportation elements and assorted storage. Testing containers such as plasticware, glassware, and surgical grade stainless steel all have different potentials.
Each of these containers has its own workable range that needs to be known and monitored.
Instruments
Avoiding contamination is only one issue. Instruments in the lab may become damaged by changes in temperature and humidity.
An accurate reading or even calibrating equipment doesn’t matter when the equipment itself is skewed. An air bubble in a lens may obfuscate vital information. A microchip suffering a short from accumulated water can ruin productivity.
Instruments are easier to monitor for constants in temperature/humidity. They face fewer environmental changes. Still, an instrument that runs constantly will have a higher operating temperature.
Maintaining your laboratory room temperature and humidity levels is a mixture of monitoring and predicting fluctuations. No environment is totally controlled. You need to adapt to changes and remove as many variables as possible.
To this end, frequent monitoring, gathering of data sets, and employing equipment that can automatically adjust for changing conditions is vital.
Even if contamination occurs, a well-established strategy allows you to find the source of the issues or be aware that the problem exists. The worst problem is not knowing that you don’t know something.
Free the Data
To maintain the validity of any research being done, you need consistent protocols across all of the laboratory testing and equipment. This starts with recording the laboratory temperature and humidity. From there, it expands to knowing variables that explain out-of-range results.
All of this is assisted by having dedicated monitors that you trust. Contact us for a full list of products and services for your laboratory monitoring needs.
Newsletter Signup - Downloadable Content
Signup for our newsletter to proceed to download.
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |

COMMENTS
To maintain humidity inside a closed place in the experiment, you must study the dew temperature, as well as the pressure, saturated pressure, as well as the nickname of evaporated water. Thank...
Here we describe OpenHumidistat: a free and open-source humidistat for laboratory-scale humidity control that is affordable (€500) and easy to build. The design is based around mixing a humid and dry air flow in varying proportions, using proportional solenoid valves and flow sensors to control flow rates.
This quick guide will help you understand the requirements around maintaining optimal laboratory temperatures and humidity. We’ll also go over the processes for maintaining optimal temperatures in the lab.
1. One option: Buy more dehumidifiers, and a space heater. Use the space heater to maintain the lab at the "elevated" temperature when the humidity is low and the dehumidifiers are not running. Another option: Run your dehumidifier when the humidity is high, but run a humidifier when the humidity is low.
Advanced systems are now able to control humidity across 5%–90% RH at a range of temperatures, allowing researchers to precisely control water vapor in the environment surrounding the sample for long periods of time.
The relative humidity is somewhat easier to control than temperature for a sample. The particulars of the ambient humidity still face changes from one lab area to another. Testing should be done within the range of typical storage and sample analysis areas to create a data set of variance.