
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons

Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

5.2: Components and Structure - Fluid Mosaic Model
- Last updated
- Save as PDF
- Page ID 12739

\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
- Describe the fluid mosaic model of cell membranes
The fluid mosaic model was first proposed by S.J. Singer and Garth L. Nicolson in 1972 to explain the structure of the plasma membrane. The model has evolved somewhat over time, but it still best accounts for the structure and functions of the plasma membrane as we now understand them. The fluid mosaic model describes the structure of the plasma membrane as a mosaic of components —including phospholipids, cholesterol, proteins, and carbohydrates—that gives the membrane a fluid character. Plasma membranes range from 5 to 10 nm in thickness. For comparison, human red blood cells, visible via light microscopy, are approximately 8 µm wide, or approximately 1,000 times wider than a plasma membrane. The proportions of proteins, lipids, and carbohydrates in the plasma membrane vary with cell type. For example, myelin contains 18% protein and 76% lipid. The mitochondrial inner membrane contains 76% protein and 24% lipid.
The main fabric of the membrane is composed of amphiphilic or dual-loving, phospholipid molecules. The hydrophilic or water-loving areas of these molecules are in contact with the aqueous fluid both inside and outside the cell. Hydrophobic, or water-hating molecules, tend to be non- polar. A phospholipid molecule consists of a three-carbon glycerol backbone with two fatty acid molecules attached to carbons 1 and 2, and a phosphate-containing group attached to the third carbon. This arrangement gives the overall molecule an area described as its head (the phosphate-containing group), which has a polar character or negative charge, and an area called the tail (the fatty acids), which has no charge. They interact with other non-polar molecules in chemical reactions, but generally do not interact with polar molecules. When placed in water, hydrophobic molecules tend to form a ball or cluster. The hydrophilic regions of the phospholipids tend to form hydrogen bonds with water and other polar molecules on both the exterior and interior of the cell. Thus, the membrane surfaces that face the interior and exterior of the cell are hydrophilic. In contrast, the middle of the cell membrane is hydrophobic and will not interact with water. Therefore, phospholipids form an excellent lipid bilayer cell membrane that separates fluid within the cell from the fluid outside of the cell.

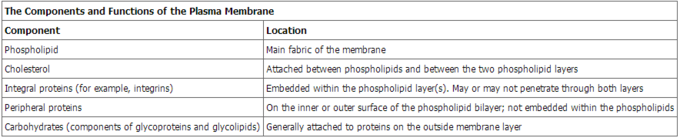
Proteins make up the second major component of plasma membranes. Integral proteins (some specialized types are called integrins) are, as their name suggests, integrated completely into the membrane structure, and their hydrophobic membrane-spanning regions interact with the hydrophobic region of the the phospholipid bilayer. Single-pass integral membrane proteins usually have a hydrophobic transmembrane segment that consists of 20–25 amino acids. Some span only part of the membrane—associating with a single layer—while others stretch from one side of the membrane to the other, and are exposed on either side. Some complex proteins are composed of up to 12 segments of a single protein, which are extensively folded and embedded in the membrane. This type of protein has a hydrophilic region or regions, and one or several mildly hydrophobic regions. This arrangement of regions of the protein tends to orient the protein alongside the phospholipids, with the hydrophobic region of the protein adjacent to the tails of the phospholipids and the hydrophilic region or regions of the protein protruding from the membrane and in contact with the cytosol or extracellular fluid.
Carbohydrates are the third major component of plasma membranes. They are always found on the exterior surface of cells and are bound either to proteins (forming glycoproteins) or to lipids (forming glycolipids). These carbohydrate chains may consist of 2–60 monosaccharide units and can be either straight or branched. Along with peripheral proteins, carbohydrates form specialized sites on the cell surface that allow cells to recognize each other. This recognition function is very important to cells, as it allows the immune system to differentiate between body cells (called “self”) and foreign cells or tissues (called “non-self”). Similar types of glycoproteins and glycolipids are found on the surfaces of viruses and may change frequently, preventing immune cells from recognizing and attacking them. These carbohydrates on the exterior surface of the cell—the carbohydrate components of both glycoproteins and glycolipids—are collectively referred to as the glycocalyx (meaning “sugar coating”). The glycocalyx is highly hydrophilic and attracts large amounts of water to the surface of the cell. This aids in the interaction of the cell with its watery environment and in the cell’s ability to obtain substances dissolved in the water.
- The main fabric of the membrane is composed of amphiphilic or dual-loving, phospholipid molecules.
- Integral proteins, the second major component of plasma membranes, are integrated completely into the membrane structure with their hydrophobic membrane-spanning regions interacting with the hydrophobic region of the phospholipid bilayer.
- Carbohydrates, the third major component of plasma membranes, are always found on the exterior surface of cells where they are bound either to proteins (forming glycoproteins ) or to lipids (forming glycolipids).
- amphiphilic : Having one surface consisting of hydrophilic amino acids and the opposite surface consisting of hydrophobic (or lipophilic) ones.
- hydrophilic : Having an affinity for water; able to absorb, or be wetted by water, “water-loving.”
- hydrophobic : Lacking an affinity for water; unable to absorb, or be wetted by water, “water-fearing.”
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

A Brief Introduction to Some Aspects of the Fluid–Mosaic Model of Cell Membrane Structure and Its Importance in Membrane Lipid Replacement
Garth l nicolson, gonzalo ferreira de mattos.
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected]
Received 2021 Nov 1; Accepted 2021 Nov 22; Collection date 2021 Dec.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( https://creativecommons.org/licenses/by/4.0/ ).
Early cell membrane models placed most proteins external to lipid bilayers in trimolecular structures or as modular lipoprotein units. These thermodynamically untenable structures did not allow lipid lateral movements independent of membrane proteins. The Fluid–Mosaic Membrane Model accounted for these and other properties, such as membrane asymmetry, variable lateral mobilities of membrane components and their associations with dynamic complexes. Integral membrane proteins can transform into globular structures that are intercalated to various degrees into a heterogeneous lipid bilayer matrix. This simplified version of cell membrane structure was never proposed as the ultimate biomembrane description, but it provided a basic nanometer scale framework for membrane organization. Subsequently, the structures associated with membranes were considered, including peripheral membrane proteins, and cytoskeletal and extracellular matrix components that restricted lateral mobility. In addition, lipid–lipid and lipid–protein membrane domains, essential for cellular signaling, were proposed and eventually discovered. The presence of specialized membrane domains significantly reduced the extent of the fluid lipid matrix, so membranes have become more mosaic with some fluid areas over time. However, the fluid regions of membranes are very important in lipid transport and exchange. Various lipid globules, droplets, vesicles and other membranes can fuse to incorporate new lipids or expel damaged lipids from membranes, or they can be internalized in endosomes that eventually fuse with other internal vesicles and membranes. They can also be externalized in a reverse process and released as extracellular vesicles and exosomes. In this Special Issue, the use of membrane phospholipids to modify cellular membranes in order to modulate clinically relevant host properties is considered.
Keywords: lipid interactions, membrane domains, extracellular matrix, lipid rafts, membrane fusion, membrane structure, cytoskeletal interactions, membrane vesicles, endosomes, membrane dynamics
1. Introduction: Cell Membranes
Cell or plasma membranes are the first cellular barriers encountered by extracellular ions, molecules, lipid vesicles and globules, viruses and other cells [ 1 ]. Cell membrane interactions with extracellular molecules determine how individual cells process nutrients, initiate cellular signaling and respond to and maintain normal cellular physiology [ 1 , 2 ]. Thus, cell membranes are important filters that provide a cellular barrier and continuity, while selectively transmitting signals, nutrients and substances from outside to inside cells and then to various cellular organelles. In addition, cells release signals and molecules to adjacent cells, tissues and distant organs, including lipid vesicles and globules containing other molecules (proteins, DNA etc.), and in doing so, they can condition host micro- and macro-environments [ 3 , 4 ]. Cells are also compartmentalized into organelles by various complex intracellular membranes that are also responsible for the biosynthesis of various molecules, energy production, replication, transportation, reutilization, destruction, secretion and other activities that are essential in cell and tissue organization and maintenance [ 3 , 4 ].
A basic concept in the organization of cellular membranes is that they are made up of amphipathic molecular components that associate into macro-structures that exclude water interactions on their hydrophobic surfaces. In contrast, the hydrophilic portions of their structures interact with the aqueous environment and other hydrophilic molecules [ 5 , 6 ]. This concept was implied by the experiments of Langmuir, who used oil layers on aqueous surfaces and measured surface tensions [ 5 ]. In 1925, Gorter and Grendel [ 7 ] used Langmuir’s methods to assess the notion that that red blood cells were surrounded by two layers of membrane lipids, which was consistent with Fricke’s estimate from cell membrane capacitance experiments that cell membranes were approximately 4 nm thick [ 8 ]. Edidin has discussed the historical concepts that cell membranes are composed of phospholipid bilayers plus some membrane proteins [ 9 ]. Using this same concept, Danielli and Davson [ 10 ] proposed that cellular membranes were lipid bilayers, as proposed by Gorter and Grendel [ 7 ], that interacted with flattened or beta-sheet proteins via the hydrophilic head groups of membrane phospholipids. Using primarily electron microscopy of erythrocytes and other cells fixed and stained with excess heavy metals, such as osmium tetroxide, Robertson visualized a trimolecular structure (protein–lipid–protein) that was named the “Unit Membrane” [ 11 ]. In contrast, a repeating subunit model of lipoproteins was proposed by Benson that did not have a matrix bilayer of phospholipids [ 12 ].
The concept that the matrix of cellular membranes contains amphipathic phospholipids that self-assemble to form lipid bilayers due to the energy provided by the hydrophobic effect and van der Waals forces has evolved over the years [ 3 , 6 , 13 ]. Integral membrane proteins assemble into this matrix and interact with membrane lipids through hydrophobic forces and much less so through hydrophilic forces between lipid head groups and hydrophilic amino acids of membrane proteins [ 3 , 6 , 9 , 13 , 14 ]. The state of membrane phospholipids is important in this process, because the membrane insertion of proteins appears to be, at least in some cases, limited to regions of membranes where a fluid lipid matrix allows protein– or protein complex–lipid hydrophobic interactions, molecular sorting and, eventually, lateral movements of membrane components [ 13 , 14 , 15 ].
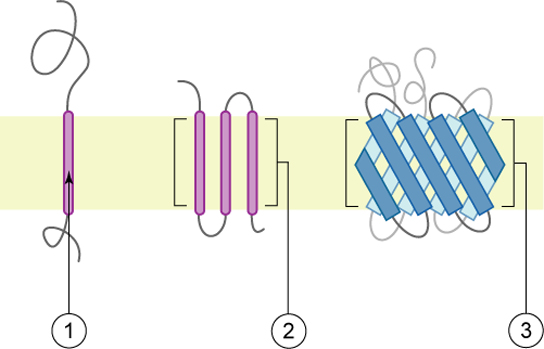
There are several different types of membrane proteins, but they can basically be assigned to three classes: integral, peripheral [ 6 , 13 ] and, introduced later, membrane-associated proteins [ 15 ]. The classic integral or intrinsic membrane proteins in the 1972 Singer–Nicolson model [ 6 ] were shown as globular in structure and bound to membranes by mainly hydrophobic forces ( Figure 1 ). The integral membrane proteins were thus intercalated into the membrane lipid bilayer and not attached to it as in previous membrane models [ 10 , 11 ]. In contrast, peripheral membrane proteins were proposed to be attached to membranes mainly by electrostatic and other forces [ 6 ]. Peripheral membrane proteins were proposed as removeable from membranes without destroying basic membrane nanostructure and continuity, and they were thought to serve as important components in providing membrane stability; curvature; scaffolding; tethering; and other characteristics, such as attachment points for enzymes and signaling complexes [ 15 , 16 , 17 ]. Later, membrane-associated proteins were added that were not generally associated with the hydrophobic matrix of membranes. Their transient interactions with membranes occurred through protein or lipid attachments instead of intercalation into a membrane lipid matrix, and their function was mainly to provide connections with other cellular components, such as enzymes, protein complexes, cytoskeletal elements and other components and structural integrity [ 3 , 15 ].
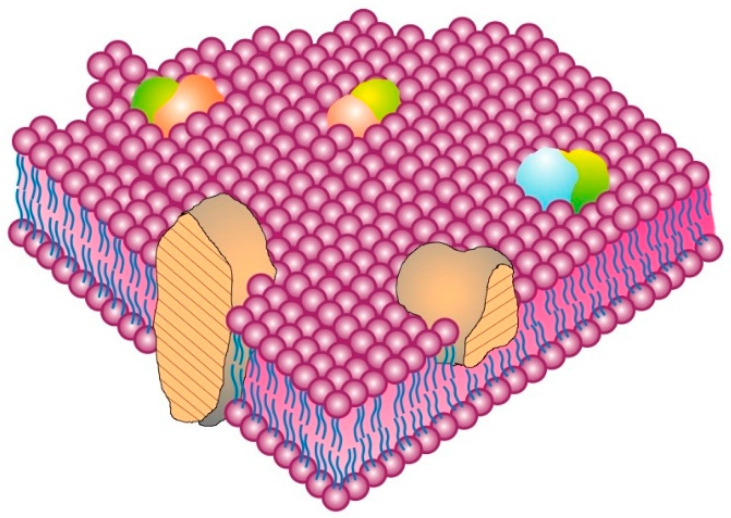
The Singer–Nicolson Fluid–Mosaic Membrane Model of cell membrane structure as proposed in 1972. In this view of a cell membrane, the solid bodies with stippled cut surfaces represent globular integral membrane proteins randomly distributed in the plane of the membrane. Some integral membrane proteins form specific integral protein complexes as shown in the figure. Integral proteins are represented in a fluid lipid bilayer. The model does not contain other membrane-associated structures or membrane domains (Modified from Singer and Nicolson [ 6 ]).
Cell membranes can be disturbed, distorted, deformed, compressed or expanded by different forces, and diverse molecules can cause these physical perturbations [ 14 , 15 , 16 , 17 ]. For example, certain peripheral membrane proteins can bind to and cause the deformation of membranes by forming crescent-shaped, helical bundles that bind to membranes via electrostatic and some hydrophobic forces, causing membrane curvature as a result of flexing and bending membranes to fit these peripheral protein structures [ 16 , 17 ]. In contrast, membrane-associated proteins, for the most part, act indirectly on membranes, usually through intermediate protein or lipid attachments. Although some membrane-associated proteins can be isolated with and loosely attached to cell membranes, they are not truly membrane proteins due to their transient interactions with membranes and their irrelevance to basic membrane nano-scale structure [ 3 , 15 ]. These membrane-associated proteins can include cytoskeletal and signaling structures at the inner cell membrane surface, or at the outer surface, they can include certain extracellular matrix and stromal components. Some cytoplasmic membrane-associated components are quite dynamic and can stabilize or destabilize cellular membranes and connect to other intracellular structures and prevent membrane components from undergoing rapid lateral movements. Alternatively, they can also be involved in translocating membrane complexes via energy-dependent contracting movements, events that can eventually lead to cell polarity, endocytosis or other cellular processes. Membrane-associated proteins are especially important in maintaining certain cellular activities, such as cell adhesion and motility, growth, endocytosis and other important cellular actions [ 3 , 4 , 15 , 18 , 19 , 20 , 21 , 22 ].
2. Fluid–Mosaic Model of Membrane Structure
The most accepted rudimentary or nanometer scale model of cell membrane structure, the Fluid–Mosaic Membrane Model, was first proposed in 1972 ( Figure 1 ) [ 6 ]. Although this is an oversimplified model that was never intended to explain all aspects of membrane structure and dynamics, it was useful in describing some of the important elements of nano-scale cell membrane architecture, continuity, cooperativity and asymmetry [ 6 , 9 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 ]. The essential elements of the Fluid–Mosaic Membrane Model have proven to be remarkably consistent with experimental results on the fundamental properties of biological membranes, but it was inevitable that the original model could not explain all of the properties of membrane structure and dynamics found in various cellular membranes [ 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 ]. For example, the concept that membrane mosaic structures or membrane domains, such as lipid rafts, as well as cell membrane-associated structures, such as actin-containing filaments, microtubules and other structures, are important in controlling membrane properties and directing the dynamics of certain cell membrane components was ascertained years after the Fluid–Mosaic Membrane Model was first presented [ 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 ]. This has resulted in completely contrary suggestions that several membrane models are necessary to explain basic membrane structure and dynamics [ 24 ] or that there is no membrane model that can explain cell membrane structure and dynamics at the nano-scale level [ 25 ]. We do not share those opinions.
With time, updates of the Fluid–Mosaic Membrane Model have made the basic representation of membrane structure far more complex, compact and much less homogeneous looking than the original scheme (cf. [ 6 ] with [ 9 , 15 , 20 , 21 , 22 , 23 ]). The newer proposals on general cell membrane structure contain additional information, such as proposals on membrane asymmetry, protein and lipid associations, membrane complexes and their dynamic segregation into various membrane domains. They can also include trans-membrane signaling complexes, cytoskeletal and stromal interactions and induced dynamic changes in membrane organization, along with other additions. These, among other changes, have now made newer cell membrane schemes much more complex and compact than the original Fluid–Mosaic Membrane Model (for example, Figure 2 ) [ 15 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 ]. Importantly, the arrangements of many membrane lipids and proteins into less freely mobile structures, such as lipid–lipid and lipid–protein membrane domains, have maximized the mosaic nature of cell membranes with less fluid areas of freely mobile membrane lipids and proteins than presented in the original Singer–Nicolson model [ 23 , 27 , 28 , 29 , 30 ]. However, the basic nano-scale organization first presented in the Fluid–Mosaic Membrane Model has generally survived, albeit in different overall organizational schemes, and the current models are certainly more crowded and complex than the original proposal [ 23 , 27 , 29 , 30 ]. To add to this complexity, Kusumi’s [ 20 , 21 ] concept of a dynamic hierarchical cell membrane organization has made an already complicated description of cell membrane organization even more complex, but necessary, in order to explain newer data on the types of mobility and restrictions of mobility of membrane components and their assembly into ever more complex structures.
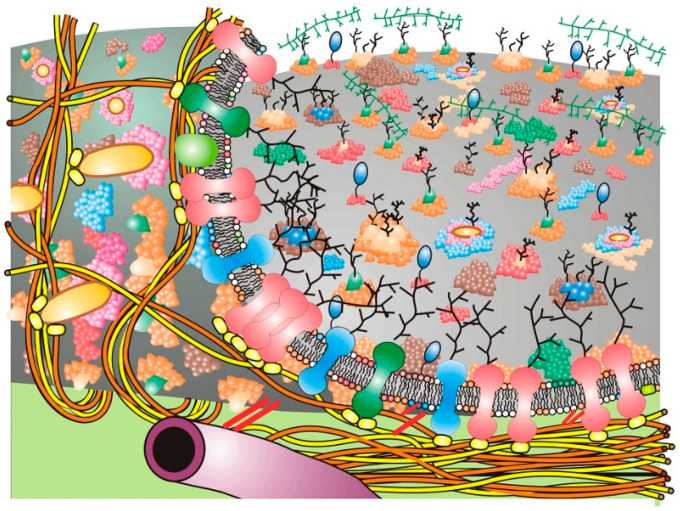
The figure represents a cell or plasma membrane that contains membrane domain structures and membrane-associated cytoskeletal and extracellular structures. The cell membrane has been pealed back at the left to reveal the bottom cell membrane surface and membrane-associated cytoskeletal elements that form barriers (corrals) that limit the lateral motions of some of the integral membrane proteins. In addition, membrane-associated cytoskeletal structures are shown indirectly interacting with integral membrane proteins at the inner membrane surface along with matrix or extracellular matrix components at the outer surface. Although this diagram presents possible schemes of integral membrane protein mobility restraint, it does not accurately represent the sizes and structures of integral membrane proteins, lipid domains or membrane-associated cytoskeletal structures (modified from Nicolson [ 23 ]).
The spontaneous, dynamic sorting of membrane components into membrane domains of specific compositions and mobilities was thought to be initially based primarily on hydrophobic and some hydrophilic interactions [ 15 ]. Such dynamic sorting avoids hydrophobic mismatches between lipids and proteins, thus preventing unsustainable membrane distortions or areas of membrane weakness [ 31 ]. The original Fluid–Mosaic Membrane Model also accounted for cell membrane asymmetry [ 3 , 16 ]. Every cell membrane studied thus far has been found to be asymmetric in terms of the display of membrane components on the interior and exterior sides of membranes [ 3 , 9 , 15 , 16 , 17 , 20 , 21 , 22 , 23 , 24 , 25 ].
An important aspect of cell membrane structure that was first hypothesized, albeit with limited evidence, in the original Fluid–Mosaic Membrane Model was the presence of oligomeric protein/glycoprotein structures in the membrane (see Figure 1 of [ 6 ] or Figures 1 and 2 of [ 15 ]). Some early evidence was the finding of different antigen distributions—dispersed [ 26 ] or micro-clustered [ 27 ]—on the same cell type. That initial notion has now become completely refined based on new evidence using state-of-the-art technology for studying the localization and dynamics of single molecules on cell surfaces at the nanometer level [ 28 , 29 ]. An important concept by Garcia-Parajo and colleagues envisions that many if not most cell membrane proteins and glycoproteins exist dynamically (on average) in small nanostructures or nanoclusters in the membrane [ 28 ]. Ma et al. have developed a method (Förster Resonance Energy Transfer sensing) that, when combined with single-particle tracking by fluorescence microscopy, can detect the intermolecular associations of neighboring proteins and their clustering events at high spatial and temporal resolutions [ 29 ]. Using this method, they were able to map the individual movements of proteins and their clustering events on live T cells and found that many receptors were already present in dense ‘nanoclusters’, and these clusters of receptors had the highest signaling efficiencies [ 30 ]. Membrane protein clustering into specific domains also appears to involve interactions with lipid domains, electrostatic interactions between proteins and lipids, inner membrane surface protein scaffolding and other properties of membrane constituents (reviewed in [ 31 ]).
3. Membrane-Associated Cytoskeletal and Extracellular Matrix Interactions
The original 1972 Fluid–Mosaic Membrane Model did not show important membrane interactions or associations with intracellular or extracellular elements [ 6 ], although subsequent modifications of this model included these types of interactions [ 15 , 20 , 21 , 22 , 23 , 24 , 25 ]. Cytoskeletal and extracellular matrix interactions are known to alter cell membrane macrostructure by restricting the dynamics or lateral movements of membrane proteins and glycoproteins, and cytoskeleton linkages are also involved in energy-dependent movements of attached membrane components and platforms [ 3 , 15 , 20 , 21 , 22 , 23 , 24 , 25 , 30 , 31 , 32 , 33 , 34 ]. Membrane linkages to cytoskeletal elements are also essential in maintaining cell polarity and overall membrane organization and dynamics [ 3 , 23 , 30 , 31 , 32 , 33 , 34 ]. Under certain circumstances, they can drive specific cellular properties, such as cell adhesion, movement, endocytosis, exocytosis and many other properties [ 3 , 23 , 28 , 29 , 30 , 31 , 32 , 33 , 34 ].
Cell membrane receptor clustering, domain formation, submembrane plaque assembly, membrane distortion and internalization, the acidification of the resulting endosomes, the degradation of endosome contents and the recycling of membrane components are all parts of the normal membrane salvaging process [ 3 , 23 ]. An important concept in more recent versions of the Fluid–Mosaic Membrane Model is that the distribution and mobility of integral membrane components can be impaired or selectively anchored by intracellular components or cell–cell, extracellular matrix and stromal interactions, resulting in cell membrane heterogeneity and cell polarity [ 3 , 9 , 15 , 20 , 21 , 22 , 23 , 24 , 25 , 32 , 33 , 34 ]. Such restraint of the mobility and dynamics of membrane components is thought to be an important element of membrane and cell physiology [ 21 , 22 , 23 , 24 , 25 , 32 , 33 , 34 , 35 , 36 , 37 , 38 ].
Cells communicate through membrane receptor complexes that can be immobilized by various interactions at the cell surface. Some communication signals are transmitted through dynamically assembled membrane-associated networks that transiently include the cytoskeleton. As mentioned above, the cytoskeleton can also generate mechanical forces that can move membrane components, membrane platforms and even entire cells or inhibit their movements, and they can help cells resist exterior mechanical forces [ 21 , 22 , 23 , 35 , 36 , 37 , 38 , 39 , 40 , 41 ]. The serial assembly of specialized cellular elements in and around membranes (integral membrane proteins and lipids, peripheral membrane proteins, adaptor proteins, cytoskeletal elements etc.) may be essential in the conversion of biochemical signals into the mechanical forces that are important in cellular behavior, tissue maintenance and cell movement [ 36 , 37 , 38 , 39 , 40 ]. Although membrane peripheral proteins have been identified as components involved in cell membrane–cytoskeletal interactions [ 20 , 21 , 22 , 23 , 37 , 38 , 39 ], membrane lipids are also important in these interactions, as well as in the formation of specialized lipid signaling domains called ‘lipid rafts’ [ 35 , 40 , 42 , 43 , 44 , 45 ]. Lipid rafts or, at least, specialized lipid domains were hypothesized years before actual experimental evidence for their existence was obtained (cf. [ 15 ] with [ 39 , 40 , 41 , 42 , 43 , 44 , 45 ]).
Specialized cell membrane domains have been proposed to be dynamic structures that can be generated by the specific interactions of bulk membrane components, extracellular ligand or ion binding, cytoskeletal interactions and other events that can result in the assembly of complex transmembrane structures or platforms [ 32 , 33 , 34 , 35 , 36 , 37 ]. An example of this is the formation of glycosylphosphatidylinositol (GPI) anchors at the cell surface in the domains or rafts that tether specific GPI-bound proteins [ 46 , 47 , 48 , 49 , 50 ]. These GPI-anchored proteins can exist in different forms, depending on the context and tissue in which they are expressed, and they are known to be involved in various cellular processes, such as cell signaling and adhesion [ 47 , 49 ]. Interestingly, individual GPI-anchored proteins display different modes of lateral movements: essentially stationary, free diffusion, anomalous diffusion and transiently confined diffusion [ 48 ]. This is discussed further in Section 5 . At the inner membrane surface, some GPI lipid domains appear to be dynamically linked to cortical actin-containing cytoskeletal structures, which may explain some of the GPI domain organization and mobilities and their spatiotemporal regulation [ 49 , 50 ]. How such nanoclusters could be involved in events such as cell spreading and possibly cell movement has been examined by Kalappurakal et al. [ 51 ]. These authors examined the role of GPI-anchored protein nanoclustering in cell spreading. They found that a membrane receptor signaling pathway directs membrane protein nanocluster formation. This occurs by the binding of Arg-Gly-Asp motif-containing ligands to the cell surface β1-integrin receptor, which eventually activates src and focal adhesion kinases, resulting in RhoA signaling. This cascade triggers actin nucleation via specific formins that, along with myosin activity, drive the nanoclustering of membrane proteins with actin-binding domains. Eventually, this cascade results in the coupling of the cell’s actomyosin machinery to inner leaflet cell membrane lipids, generating functional GPI-anchored protein nanoclusters that are involved in cell spreading [ 49 , 51 ].
Macromolecular membrane complexes on the cell surface can also recruit specific peripheral and membrane-associated proteins at the inner cell membrane surface to form transmembrane domains, platforms or plaques that are competent for initiating cellular signaling via structural or enzymatic processes or undergoing further attachments to cytoskeletal elements [ 47 , 48 , 49 , 50 , 51 ]. This can result in membrane reorganization, immobilization, signaling events or internalization in endosomes [ 21 , 22 , 23 , 24 , 47 , 48 , 49 , 50 , 51 ]. We have to consider that cell membranes are essentially completely integrated mechano-structures that exist within single cells, groups of cells and tissues [ 3 , 23 , 33 , 34 , 35 , 36 ]. Cell membranes are continuously interacting with and linking to various structural components inside and outside cells while receiving signals and contact information from the microenvironment, and they filter and pass these signals on to stimulate appropriate cellular responses. Cell membranes also send messages into the extracellular environment, maintaining cell polarity, cell mechanical properties and tissue barriers while undergoing constant turnover and reassembly of their components.
Over time, the basic nano-scale organization of cell membrane models has evolved significantly from the original models of a rather homogeneous-looking structure ( Figure 1 ) [ 6 ] into models that are dynamic yet contain mosaic structures that comprise specific dynamic domains of varying sizes, compositions and mobilities and that can transform into specific membrane regulatory and mechanical structures or platforms that are then linked to various intra- and extra-cellular components [ 23 , 24 , 25 , 34 ]. In Figure 2 , a rather simplified schematic of these additions to the Fluid–Mosaic Model is depicted, but one should not take such schemes too seriously, because they shall surely change again over time as more information comes to light.
4. Membrane Component Interactions in Cell Membranes
One of the more obvious properties of cell membranes that was not adequately portrayed in the original Fluid–Mosaic Model is that membrane constituents are, in general, nonrandomly distributed. For example, membrane lipids are now accepted as being asymmetrically dispersed on the inner and outer leaflets of plasma membranes and also unevenly distributed in the membrane plane [ 3 , 15 , 16 , 17 , 23 , 25 , 31 , 52 , 53 , 54 ]. In addition to membrane phospholipids, non-phospholipid membrane lipid molecules are also distributed nonrandomly. For example, cholesterol can affect membrane lipid distributions, and cholesterol is often found enriched in specific membrane domains [ 53 , 54 , 55 ]. Cholesterol distribution is thought to be due, in part, to its affinity for both the fluid and solid phases of membrane lipids [ 42 , 43 , 44 , 45 ]. Cholesterol partitions into liquid-ordered and disordered phases to roughly the same extent, but this partitioning can differently modify the properties of these dissimilar membrane lipid phases [ 54 , 55 ]. Lipids can also modify certain physical properties of membranes [ 56 ]. For example, ceramides and lysophospholipids are known to induce membrane curvature, while other lipids, such as cholesterol, can modify membrane lateral elasticity [ 52 , 54 , 55 ].
Specific membrane lipids, for example, sphingolipids, are important in the formation of ordered membrane lipid mosaic domains or ‘lipid rafts’ [ 42 , 43 , 44 , 45 ]. Along with phosphatidylcholine, sphingomyelins constitute more than one-half of the plasma membrane phospholipids and form the most important partners for cholesterol in lipid rafts and other specialized lipid domains. Such small, ordered membrane raft domains that are formed by the preferential associations of cholesterol and saturated lipids are generally surrounded by liquid-phase lipids, and they are thus capable of membrane lateral mobility [ 42 , 43 , 44 , 45 ]. They can also selectively recruit other lipids and proteins into their structures [ 40 , 54 , 56 ]. The lipids within such lipid mosaic domains are not fixed in place—they are still dynamic and can slowly exchange with bulk membrane lipids, as well as with lipids in other membrane domains. In terms of overall size, lipid domains, such as lipid rafts, are usually less than 300 nm in lateral diameter, and most are mostly within 10–200 nm in diameter [ 57 , 58 , 59 , 60 ], but some can be larger, and they can undergo clustering induced by protein–protein and protein–lipid interactions into micrometer sized (>300 nm in diameter) domains [ 39 , 40 , 60 ].
As mentioned above, lipid domains can also contain some integral and peripheral membrane proteins, and these mixed domains can also change in composition with time. The proteins and glycoproteins that are sequestered into membrane domains or rafts can turn these membrane domains into functional signal transduction platforms that are coupled across the membrane and can initiate immune signaling, host–pathogen interactions, endocytosis, cell death regulation and many other events [ 35 , 44 , 60 ]. In addition, membrane proteins can have profound effects on membrane lipids. They can locally deform membranes and cause the reorganization of membrane lipids to form new membrane domains, as well as regulate membrane properties, such as charge density and diffusion rates [ 61 ].
When integral membrane proteins interact with membrane lipids within cellular membranes, portions of their structures must directly interact with the acyl chains of membrane phospholipids or the hydrophobic portions of these and other membrane lipids. This is accomplished by the concept of hydrophobic matching between different classes of membrane molecules [ 52 , 53 , 56 ]. The concept of hydrophobic matching between the hydrophobic core of the lipid bilayer and hydrophobic stretches of amino acids in integral membrane proteins is essential for the formation of stable membrane structures. If the hydrophobic portions of their structures are mismatched, an elastic distortion of the lipid matrix around the integral membrane protein can occur [ 52 , 53 ]. This can induce protein conformational changes that can affect protein function and protein–protein interactions. Such membrane proteins can also aggregate to cause super-domains to form in membranes. To add to this, there are other physical forces, such as lateral pressure forces, lateral phase changes, membrane curvature and ionic interactions, among other forces, that are important in determining membrane structure [ 62 , 63 ].
5. Restrictions on Membrane Mobility and Membrane Domains
There is ample evidence for various restrictions in the rotational and lateral mobilities of certain membrane components and their residence in or compartmentalization into membrane domains in which there are changes in local compositions, lateral organization and dynamics that are different from the average membrane organization [ 23 , 40 , 45 , 64 ]. For example, the lateral movements of integral membrane proteins in the membrane plane are often restricted by multiple cis- and trans-membrane interactions that constrain movements within specific membrane domains [ 20 , 21 , 22 , 23 , 24 , 25 ]. These include extracellular interactions, such as binding to the extracellular matrix and stroma; the formation of specialized membrane domains (lipid rafts and larger, more heterogeneous domains); and integral protein–glycoprotein complexes. At the inner membrane surface, peripheral membrane barriers, curvature-causing peripheral membrane proteins, cytoskeletal interactions and other obstacles can also control membrane component movements [ 15 , 20 , 21 , 22 , 23 , 24 , 25 , 28 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 ]. To accommodate the many diverse structures and interactions that can occur at the inner and outer cell membrane surfaces, cell membranes must contain a number of membrane domains, barriers, platforms and membrane-associated structures [ 15 , 20 , 21 , 22 , 23 , 24 , 25 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 63 , 64 , 65 ].
With time, all of the current basic models of the cell membrane structure evolved to be more complex than the original Fluid–Mosaic Model, and they also contain additional membrane-associated structures and domains that were impossible to contemplate at the time of the original Fluid–Mosaic proposal [ 6 ]. Since these structures were discovered well after the original model was proposed, they are missing in the original Fluid–Mosaic Model [ 6 ]. The one aspect that all of the current membrane models have in common is that they are remarkedly more complex than the original Fluid–Mosaic Model, but they still retain some of the basic elements of the original model [ 20 , 21 , 22 , 23 , 24 , 25 , 28 , 31 , 34 , 40 , 62 ].
What is important in current membrane model proposals is that the restraint of mobility of integral glycoproteins or glycoprotein receptors in the cell membrane plane and their presence in specific membrane domains has functional physiological consequences. This concept has attracted an immense amount of attention in the last two decades [ 20 , 21 , 22 , 23 , 24 , 25 , 37 , 40 , 42 , 43 , 44 , 45 , 60 , 61 , 62 , 63 , 64 ]. The lateral movements of some specific membrane proteins and cell surface receptors have been examined, and their movements (or restraint of movements) have been organized into various categories. Some examples of these categories are the following: ( a ) random movement or free diffusion in the fluid portions of the membrane; ( b ) transient movements confined by membrane obstacles made up of protein clusters that have been likened to ‘fence posts’ or ‘pickets’; ( c ) transient movements that are constrained by structural domains or ‘corrals’ circumscribed by cytoskeletal elements and their attachment molecules; and ( d ) directed movements due to attachment to and contraction of the cytoskeleton [ 20 , 21 , 22 , 23 , 24 ]. This has led to descriptions of the various two-dimensional motions of membrane components within and between various membrane domains as ( i ) free Brownian diffusion; ( ii ) anomalous diffusion caused by changes in the lipid nano-environment; ( iii ) channeled diffusion defined by membrane-associated cytoskeletal structures; ( iv ) confined diffusion limited by defined structural ‘corrals’; and ( v ) hop diffusion, where diffusion occurs intermittently and differently between dissimilar domains [ 20 , 21 , 22 , 64 ]. Thus, the original description of integral membrane proteins freely diffusing in the membrane plane without regard to different membrane domains or obstructions is limited to only one of these categories [ 6 ]. Moreover, the original Fluid–Mosaic proposal of cell membranes did not adequately describe the multiple ways that membrane components can aggregate, separate, move or be restrained from movement in various domains in the plane of the membrane, nor did it describe the types of molecular interactions that can control membrane dynamics [ 20 , 21 , 22 , 23 , 24 , 25 , 39 , 40 , 49 , 55 , 64 ].
Our current concept of cell or plasma membrane dynamics is that substantial portions of integral membrane proteins are in mosaic structures that are incapable of free lateral diffusion in the cell membrane plane, or they may only be transiently available to undergo free movements in the membrane plane [ 20 , 21 , 22 , 23 , 24 , 34 , 36 , 47 , 48 , 64 ]. Many cell membrane components are thought to be confined, at least part of the time, to membrane domains circumscribed by barriers within the membrane matrix or barriers attached to membranes, such as cytoskeleton networks, or at the outer surface by interactions with the extracellular matrix or stroma [ 20 , 21 , 22 , 23 , 24 , 25 , 34 , 35 , 36 , 40 , 49 , 55 , 60 , 64 ]. Since cell membranes are dynamic structures, over time, some integral proteins and, separately, some lipids can escape from one or more of these domains and move to adjacent domains. Alternatively, they can escape membrane domains altogether, or they can undergo associations in the membrane plane and become super-sized mosaic structures, preventing extra-domain movements [ 20 , 21 , 22 , 23 , 24 , 25 , 60 , 64 ]. These latter super-sized structures may also be precursors of endosomes brought into cells by endocytosis mechanisms or exosomes released from plasma membranes. The abilities of membrane lipids and proteins/glycoproteins to move between adjacent membrane domains may be related to the extent of their aggregation with similar or different components, the sizes of membrane and cytoplasmic barriers to movements and the complex interactions of these barriers with the cytoskeleton and extracellular matrix [ 20 , 21 , 22 , 23 , 39 , 40 , 48 , 49 , 64 ]. In the latter case, mucin polymers and long-chain polysaccharides can generate entropic forces that favor or disfavor the projection of cell extensions from the cell surface, as well as control cell shape and immobilize certain cell surface components [ 65 , 66 ].
The actual sizes of membrane domains can vary quite dramatically, depending on domain composition and other factors, from small lipid-only domains or small lipid rafts to rather large, complex glycoprotein–lipid domains that can also have linkages to other structures. Kusumi et al. [ 20 , 21 , 67 ] have estimated the approximate diameters of various membrane domains, such as micro- and sub-micrometer-sized domains. They are thought to vary in area from 0.04 to 0.24 μm 2 , with approximate transit times of some membrane glycoprotein receptors in such domains ranging from 3 to 30 s. They propose that smaller membrane domains, such as nano- or meso-sized domains in the range of 2–300 nm in diameter, are also present, with complex actin-containing cytoskeletal fence domains in the range of 40–300 nm in diameter compared to entirely lipid raft domains that are usually in the range of 2–20 nm in diameter. Moreover, there are dynamic integral membrane protein complex domains that can vary in size with a minimum range of 3–10 nm in diameter and containing only a few components and a maximum size of at least one-hundred times this diameter [ 20 , 21 ]. The presence of so many different types of cell membrane domains and the selective presence of membrane protein receptors in some of these domains indicate that there is another level of membrane compositional and organizational complexity beyond the original description of the Fluid–Mosaic Membrane Model. This has been called hierarchical membrane organization by Kusumi and his colleagues [ 20 , 21 ].
The proposal that cell membranes are organized into complex hierarchical structures is based on several observations on the variability and dissimilarity of the lateral motions of various cell surface receptors and other membrane components and the ability of cells to quickly reorganize their cell surface membrane structures in order to respond to intracellular and extracellular signals [ 20 , 21 ]. This type of dynamic organization may have evolved so that cells can rapidly respond to ligand binding and other signals. In addition, it may be more efficient to have receptors pre-positioned in the cell membrane within signaling domains so that they can undergo more rapid and specific aggregation into supramolecular transmembrane signaling structures [ 21 ]. The presence of barriers or ‘fences’ on the inner plasma membrane surface that limit the lateral motions of specific integral membrane protein components within cytoskeletal-fenced ‘corrals’ or tethering them directly or indirectly to membrane domains may create relatively stable, local membrane domains of high receptor densities. The Kusumi-type hierarchical structures incorporate membrane domains with cell surface receptor diffusion rates 5 to 50 times slower compared to the same components when they are free to diffuse laterally. Over time, such receptors are generally thought to be confined to the specific membrane sub-regions with restricted mobilities [ 20 , 21 ]. This type of organization has been described as important in allowing the pre-positioning of receptors so that they are more fully capable of responding quickly, efficiently and specifically to an appropriate extracellular signal, especially if this type of signal transmission involves the formation of complex signaling platforms [ 21 ].
Some fundamental requirements of cell membrane signaling via receptor–ligand binding are thought to consist of a basic Fluid–Mosaic Membrane structure plus various specific membrane nano- and micro-sized domains or compartments capable of forming larger clusters, surrounded by fluid-phase lipids, which can be dynamically trans-membrane linked to cytoskeletal systems [ 21 , 22 , 23 , 64 ]. A membrane signaling compartment or signaling domain can be further defined by whether aggregation with similar or different domains occurs as well as their confinement by cytoskeletal fencing or protein ‘fenceposts’ or other properties. Various membrane barriers may be used to prevent large-scale coalescence of smaller membrane domains [ 20 , 21 ].
A variety of different membrane domains and structures probably exist in cell membranes in order to accommodate the large number of possible extracellular signals that cells receive so that particular signals can be distinguished from one another. A facsimile of a Fluid–Mosaic membrane containing lipid raft domains, glycoprotein domains, barrier or ‘corral’ domains and other membrane-associated structures is depicted simplistically in Figure 2 [ 23 ]. One should not take such schemes too seriously, because they will likely undergo further changes when new information and data are available. How membrane structures can be affected by domain-clustering agents, such as the binding of various extracellular molecules, changes in ion concentrations and, especially, the integration of lipid molecules from outside the cell in order to change cell and tissue and ultimately host responses, will be the subject of this Special Issue.
In contrast to the very dynamic membrane domains involved in cell signaling, nutrient transport and other properties, cells must also have less dynamic, more stable mosaic membrane structures that are involved in maintaining cell polarity, stable cell–cell interactions and tissue organization. These latter properties of cell membranes require mosaic structures that are not unusually mobile but are more stable, less mobile and integrated and linked to extracellular structures in the pericellular spaces between cells. Such structures could also be (and are often) transmembrane linked to cytoskeletal elements and peripheral membrane networks inside cells to create a fully integrated tissue structural support system [ 22 , 23 , 25 , 33 , 34 , 35 , 36 , 65 ].
6. Membrane Vesicles, Globules and Membrane Fusion
Cells are highly dependent on their abilities to capture nutrients and remove cellular waste, such as toxic and damaged molecules. They must transport and transfer various molecules between the extracellular and intracellular microenvironments and between the various organelles and cellular compartments. Cells have to rapidly move various nutrients, structural components and newly synthesized molecules to where they are needed intracellularly and to remove them if they are damaged, degraded or no longer needed. The biosynthesis of molecules inside cells that will eventually be sent to various cell organelles, sub-organelle compartments, the plasma and other membranes, or secreted to the extracellular microenvironment, is generally followed by their immediate binding to specific transport molecules or their packaging into small membrane vesicles that are delivered to specific intracellular membranes or plasma membrane domains at specific membrane contact sites. Alternatively, different intracellular membranes can undergo contact and transient fusion to deliver membrane constituents [ 68 , 69 , 70 , 71 ]. Such processes are also used to repair damage to the plasma and intracellular membranes by removing damaged molecules in order to maintain cell function [ 70 , 71 ].
Membrane components, such as membrane lipids, are often moved around and within cells using carriers, such as lipoproteins and lipid-binding proteins, as well as small lipid globules, membrane vesicles and, of course, as mentioned above, intracellular membranes. The carrier membrane vesicles or intracellular membranes eventually fuse with target membranes to deliver their cargo. Periodic membrane–membrane fusion events occur naturally in cells in order to redistribute membrane lipids and other components between different cellular compartments and remove damaged membrane constituents [ 3 , 69 , 70 , 71 , 72 , 73 , 74 ]. Membrane fusion ensues during a variety of normal cellular events, such as fertilization, development, endocytosis, secretion, nerve transmission and many other normal developmental and restorative processes. They are also important in many pathologic situations, such as infection, inflammation, neoplasia, cell death and other events [ 72 , 73 , 74 ].
Inside cells, directed lipid and vesicle transport and their membrane fusion events depend, in large part, on membrane lipid properties, such as the composition, distribution and acylation of lipids in the transport vesicles and in the target membrane domains, as well as the presence of targeting receptor proteins and specific electrolytes at the point of fusion [ 69 , 75 , 76 , 77 , 78 ]. Thus, lipid composition is important in transport vesicles and membranes, as well as in specialized membrane domains that are the targets of fusion events. For example, in some transport systems, sphingolipids are found to be concentrated in vesicles destined to fuse with plasma membranes at the sites of specific membrane domains [ 76 , 77 , 78 , 79 , 80 ]. The presence of specific polyphosphoinositides with their tethered proteins is also important in directed lipid vesicle-mediated transport to the exterior cell surface [ 76 , 80 , 81 , 82 , 83 ]. Once these membrane transport vesicles attach to target membrane domains, membrane fusion follows, which is dependent on the presence of specialized membrane-binding fusion machinery. This fusion machinery requires specific ‘fusogenic’ proteins that pull adjacent membranes together and electrolytes, such as calcium ions, to promote lipid bilayer fusion [ 71 , 73 , 79 , 80 ]. The process requires ( i ) the close apposition of the membranes, while counteracting the electrostatic forces between the outer layers of the lipids that will fuse; ( ii ) the destabilization of the bilayer lipid structure, allowing the incorporation of lipids in a non-bilayer transition structure; and ( iii ) the transient reunification into a bilayer lipid structure [ 76 , 79 ].
At the cell membrane inner surface, the presence of membrane fusogenic proteins appears to be essential for directing the fusion process. In plasma membranes, this has been understood to constitute a specialized dynamic membrane microdomain involved in secretion called a ‘porosome’ [ 76 , 81 ]. In some normal cells, porosomes appear as ‘pits’ measuring approximately 0.5–2 μm in diameter with depressions of 100–180 nm in depth [ 81 ]. They are involved in directing exocytosis to particular sites at the cell surface. Porosomes are necessary for various normal functions in cells, such as the secretion of proteins, glycoproteins, enzymes, bioregulators and other important molecules [ 81 , 82 ]. Cells also secrete components by releasing or shedding intact plasma membrane microvesicles [ 83 , 84 , 85 , 86 , 87 , 88 , 89 ].
As mentioned above, membrane lipid transport occurs inside cells when lipids are attached to carrier proteins or when they are present in micelles, vesicles, globules, membranes and other transport forms [ 68 , 69 , 70 , 71 , 77 , 80 , 81 , 82 , 83 , 84 , 85 ]. These lipid transport systems usually function on a ‘bulk flow’ or ‘mass action’ basis, where the sources that contain higher concentrations of certain membrane lipids can deliver excess lipids to membranes that have lower concentrations of these particular lipids [ 82 ]. Once membrane lipids are transported to their destinations, they can also be enzymatically modified in order to reflect the compositions of the membranes at their final destinations [ 70 , 80 , 82 ]. The bulk flow or mass action delivery of glycerolphospholipids (GPLs) to particular membrane sites may also be used to remove oxidized or damaged lipids from membranes and eventually degrade them or export them from cells [ 71 , 81 , 82 ].
Cells almost continuously release small 0.1–2 μm diameter membrane vesicles that are derived from budding plasma membranes that extend into the extracellular environment [ 3 , 81 , 83 , 84 ]. This is very apparent in cells in tumors, where the transformed cells appear to release many of these vesicles continuously. The released tumor cell vesicles appear as small (<100 nm diameter) microvesicles called exosomes [ 3 , 83 , 84 , 85 , 86 , 87 , 88 , 89 ]. The tumor cell exosomes also contain various non-membrane molecules, such as small DNAs, RNAs, proteins, enzymes, biomodulators, receptors and other molecules [ 85 , 86 , 87 , 88 , 89 ]. The release of small vesicles from normal and tumor cells and their arrival at near and distant cells may constitute a form of cell–cell communication between similar or different cells. This type of membrane exchange of information between cells is not unique. Vesicles released from normal cells have been found in virtually every extracellular fluid, where they appear to play a role in normal cell communication and in the regulation of inflammation, coagulation, development and other normal physiological processes [ 85 , 86 , 87 , 88 ]. In tumors, the released membrane vesicles may affect tumor cell–cell interactions as well as tumor–normal cell interactions in the microenvironment, and this has been proposed to promote or at least affect tumor progression, angiogenesis, invasion and metastasis [ 3 , 74 , 86 , 87 , 88 , 89 ].
Extracellular membrane vesicles, exosomes and other intracellular membrane vesicles can be involved in signaling changes in intracellular levels of calcium, variations in membrane phospholipid content, changes in cellular energy production and many other responses. They can also signal changes in the regulators that can affect cytoskeleton–membrane interactions; membrane-acting enzymes; other effectors of exocytosis, hypoxia and oxidative events; and responses to hydrodynamic stress [ 85 , 86 , 87 , 88 ].
7. Membrane Lipid Replacement with Dietary Phospholipids
Plasma and intracellular membrane physiology and function depend on the integrity of every category of cellular membrane component, and especially their most venerable components, including unsaturated membrane phospholipids, which are especially susceptible to oxidative damage. When membrane lipids, especially membrane phospholipids, are damaged, degraded or destroyed, they must be repaired or replaced to maintain normal cellular functions and physiology [ 70 , 71 , 82 , 90 , 91 , 92 , 93 , 94 ]. The dietary replacement of damaged membrane phospholipids with undamaged, functional membrane phospholipids is essential for maintaining cellular and tissue functions and for general health [ 90 , 91 , 92 , 93 , 94 ]. To maintain fully functional cellular membranes, animals and especially humans must consume a diet rich in membrane precursors, such as phospholipids and other lipids, proteins, minerals, carbohydrates and other metabolites. This can be difficult to achieve, especially during chronic and acute illnesses; hence, dietary supplements containing membrane phospholipids have been used to supplement and maintain general membrane health [ 90 , 91 , 92 , 93 , 94 ]. Moreover, many of the most sensitive molecules that make up cellular membranes, especially polyunsaturated GPLs, are quite susceptible to oxidative damage and degradation, and membranes unprotected from oxidative and free radical injury have limited half-lives and must be constantly replaced. When orally ingested as foods, membrane phospholipids can be degraded before ingestion or modified within the gastrointestinal system prior to absorption, as well as during transport to cellular destinations, or they can be taken in as intact molecules without degradation or modification. For example, in the gastrointestinal system, excess membrane GPLs can be absorbed as small phospholipid globules and micelles with their constituents basically undegraded, or they can be absorbed in a more specific but less efficient process that utilizes carrier or transfer molecules [ 68 , 69 , 70 , 71 , 90 , 91 , 92 , 93 , 94 ]. Overall, the process is usually very efficient, and over 90% of ingested phospholipids are normally absorbed and transported into the blood within hours after entering the gastrointestinal system [ 95 ]. While in the blood circulation, membrane phospholipids are usually associated with carrier molecules, such as lipoproteins, or the cell membranes of blood cells. However, when they are present in excess amounts compared to fasting levels, they can also be present in small phospholipid globules or micelles. Eventually, they are transported to tissues and cells, where they are transferred by direct membrane contact, endocytosis or by specific carrier and transport proteins into cells. Once inside cells, membrane phospholipids can be moved to various cellular compartments by a number of mechanisms, including membrane–membrane transfer, carrier molecules, small membrane vesicles, lipid globules and chylomicrons, among other mechanisms, to various cellular and organelle membranes [ 68 , 77 , 80 , 81 , 82 , 90 , 91 , 92 , 93 ].
Dietary membrane phospholipids, such as GPLs from a variety of plant and animal sources, can be enzymatically modified before, during and after their delivery to cells by exchange or modification of their head groups and fatty acid side chains to reflect the specific compositions of their destination membranes. After they have been exchanged or partitioned into their target membrane sites, GPLs and other membrane lipids can be further enzymatically modified to reflect the unique and ever-changing structural and functional membrane needs of various organelles and cells. The entire process of membrane lipid uptake, transport, replacement, exchange and removal is driven overall by a ‘mass action’ or ‘bulk flow’ process [ 82 ]. Thus, when particular membrane phospholipids are in great excess during the dietary uptake process, they have an advantage in being able to reach their final cellular destinations with significantly less degradation or free radical oxidation. This ‘mass action’ basis of bulk membrane lipid uptake and transport is also true of the reverse of this process, which eventually results in the exchange and removal of damaged, oxidized phospholipids and their elimination via the gastrointestinal system [ 69 , 70 , 71 , 80 , 82 ].
8. Membrane Lipid Replacement in Aging and Chronic Illnesses
Membrane Lipid Replacement (MLR), the use of oral dietary supplements containing essential polyunsaturated GPLs and other membrane lipids, has been successfully used to maintain and recover lost or diminished organelle and membrane function. The most common therapeutic use of oral MLR phospholipids is to treat fatigue and improve mitochondrial function [ 90 , 91 , 92 , 93 , 94 ]. Fatigue is the most common complaint of patients seeking general medical care, and it is associated with aging and most if not all chronic and many acute medical conditions [ 95 , 96 ]. Fatigue is considered a complex, multi-dimensional sensation that is not completely understood at the molecular level, but it is perceived to be associated with a loss of overall energy, extreme mental or physical tiredness, a feeling of exhaustion or diminished endurance or loss of function and an inability to perform even simple tasks without exertion. In aged individuals and in chronic and most acute diseases, fatigue is present due to a variety of causes [ 90 , 92 , 93 , 96 , 97 ]. In patients with moderate to severe fatigue complaints, fatigue has been directly related to the loss of mitochondrial function and diminished production of mitochondrial ATP [ 98 ].
In chronic fatiguing illnesses, such as chronic fatigue syndrome and myalgic encephalomyelitis (CFS/ME), long-term fatigue is the most obvious symptom present, or the dominant symptom, and is usually the primary patient complaint [ 97 , 98 , 99 , 100 ]. Moreover, in almost all chronic illnesses, fatigue is a common primary or secondary complaint [ 93 , 97 , 98 , 99 , 100 ]. Along with aging, advanced cancers and other diseases, chronic fatigue is a major symptom or complaint. Although severe fatigue is usually related to significant loss of mitochondrial function, mild fatigue is not necessarily related to the loss of mitochondrial function. Mild fatigue can be found in depression and in some psychiatric conditions that are not always related to the loss of mitochondrial function [ 98 , 100 ].
MLR has been used for general health, aging and the treatment of various clinical conditions using oral, antioxidant and environmentally protected GPL [ 90 , 91 , 92 , 93 , 94 , 96 ]. The oral use of protected GPL has been successfully used for significantly reducing fatigue in patients with chronic fatigue and other chronic illnesses, including CFS/ME, fibromyalgia and other fatiguing illnesses (reviews: [ 90 , 91 , 92 , 93 , 96 ]). For example, in a cross-over clinical study on the effects of oral NTFactor ® , a mixture of protected GPL, pre- and pro-biotics and other ingredients was used to treat chronic fatigue symptoms in moderately to severely fatigued aged subjects (61–77 years old). It was found that there was good correspondence between the patients’ reductions in fatigue scores (reductions of 35–43%) and their improvements in mitochondrial function tests [ 101 ]. This cross-over clinical study indicated that aged individuals with moderate to severe chronic fatigue benefited significantly from taking the MLR oral GPL supplement. They showed significant improvements in fatigue and other clinical parameters during the test arm of the study, but these were slowly reversed when patients were switched to placebo. Their mitochondrial function, as measured by their mitochondrial inner membrane trans-membrane potential, matched the clinical data and showed enhancement up to 45% while on the MLR supplement, but this was significantly reduced after the patients were switched to placebo. When on the MLR supplement, their mitochondrial function tests were similar to results found in much younger subjects (average age of 31 years old), but only if they continued to take the MLR oral supplement [ 101 ]. Similar positive results on the effects of the MLR GPL supplement NTFactor ® or NTFactor Lipids ® on reducing fatigue were found in various patients with chronic fatigue syndrome (CFS/ME), fibromyalgia, Gulf War illness, chronic Lyme disease and various cancers, with reductions in fatigue ranging from 26% to 43% [ 90 , 92 , 93 , 96 , 102 , 103 , 104 , 105 ].
9. Membrane Lipid Replacement in Pain Reduction and TRP Channels
MLR supplements, such as NTFactor Lipids ® , have been used to help reduce a variety of symptoms, including pain, peripheral neuropathy and gastrointestinal symptoms, in chronically ill patients [ 96 , 102 , 105 , 106 ]. Pain is a complex phenomenon that can be caused by injury or illness, and it is usually classified according to different criteria based on its pathophysiological mechanism, duration, etiology and anatomical source [ 107 , 108 ]. One type of pain, nociceptive pain, has been described in acute or chronic forms as a sharp or throbbing pain that is often experienced in the joints, muscles, skin, tendons and bones. Nociceptive pain is considered a short-lived condition, although it can also be chronic, generated by the body in response to potentially harmful stimuli, generating reflexes that hypothetically protect the individual from potential damage. This type of pain can be divided into two categories: somatic nociceptive pain, which is usually localized in the dermis, and visceral nociceptive pain, which usually arises as diffuse and poorly defined pain sensations in the midline of the body [ 108 , 109 ]. Multiple events can act on nociceptors to induce pain, and the membrane channels that are involved in nociceptive pain have been identified as transient receptor potential (TRP) channels (TRPV1, TRPM3, TRPA1 etc.) [ 110 ]. This large family (>50 subtypes) of membrane channels has been a recent therapeutic target for developing new treatments of chronic pain [ 111 ].
In mammals, the TRP channel superfamily consists of 6 subfamilies and 28 members that mainly act as cation channels. TRP channels possess a primary structure that is common to all members, consisting of six trans-membrane domains and one hydrophilic loop that forms the pore that is permeable to monovalent cations and, in certain cases, calcium ions [ 112 ]. Certain TRP channels are critical for nociception and thermal sensitivity [ 98 , 99 ]. Previously, it was found that some membrane channels required the presence of GPL phosphoinositides for activity [ 113 , 114 , 115 ]. TRP channels are regulated by membrane GPL, such as phosphatidylinositol 4,5 bisphosphate or PI(4,5)P2, where the GPL can act as an agonist with desensitization properties, though initially it was described as a general inhibitor of TRP channels. For example, PI(4,5)P2 can inhibit the heat- and capsaicin-activated TRPV1 channels, and the breakdown of this lipid upon phospholipase C activation relieves this inhibition, resulting in the potentiation of TRPV1 activity by pro-inflammatory agents, such as bradykinin [ 116 ]. Even if the TRP channels are activated by PI(4,5)P2, they quickly become unresponsive as they become desensitized, losing the ability to be stimulated [ 117 ]. Phospholipase C can also catalyze the hydrolysis of PI(4,5)P2, resulting in the formation of the two classical second messengers: inositol 1,4,5 trisphosphate (IP3) and diacylglycerol (DAG). The general view is that the negatively charged head group of PI(4,5)P2 interacts with positively charged residues in the cytoplasmic domains of the TRP channels [ 118 ]. This was later confirmed when the co-crystal structures of TRP channels with and without PI(4,5)P2 were published [ 119 ]. Though the specific mechanism of action of phosphoinositosides on TRP nociceptor channels is not fully understood (inhibition or activation with desensitization), both proposed mechanisms lead to a final decrease in the activity of these channels either by inhibition or desensitization. Thus, the final result is a decrease in pain sensitivity and nociception promoted by GPL, which is present in MLR supplements, such as NTFactor Lipids ® [ 90 , 92 ].
The requirement of higher doses of GPL mixtures to inhibit pain (usually 6 g or more per day of the MLR supplement NTFactor Lipids ® ) may be explained by the fact that PI and its derivatives are not major constituents of MLR supplements [ 90 , 92 ]. Most patients on MLR supplements, such as oral NTFactor Lipids ® , gradually move to higher daily doses of the MLR supplement to maintain pain control [ 106 ]. Currently, we are examining the possible mechanism(s) whereby membrane GPLs alter nerve membrane depolarization and other important properties involved in pain transmission.
10. Final Comment
The overwhelming amount of basic information on membrane structure, organization and dynamics, briefly presented here as an overview, has been accepted by the scientific community. However, we are just beginning to understand the role of intracellular membranes and cell membrane properties in explaining various biological phenomena, such as pain. This information will be essential in explaining the complex relationships between cells in tissues and for the eventual development of new therapeutic approaches to overcome various pathological conditions.
G.L.N. acknowledges support from the Institute for Molecular Medicine and Nutritional Therapeutics, Inc. G.F.M. has support from Research and Development Funds CSIC 91 and 137 from Universidad de la República, Montevideo.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
G.L.N. is a part-time consultant to Nutritional Therapeutics, Inc. No other possible conflicts of interest are reported.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- 1. Powell D.W. Barrier function of epithelia. Am. J. Physiol. Gastrointest. Liver Physiol. 1981;241:G275–G288. doi: 10.1152/ajpgi.1981.241.4.G275. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Lande M.B., Priver N.A., Zeidel M.L. Determinants of apical membrane permeabilities of barrier epithelia. Am. J. Physiol. Cell Physiol. 1994;267:C367–C374. doi: 10.1152/ajpcell.1994.267.2.C367. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Nicolson G.L. Cell membrane fluid–mosaic structure and cancer metastasis. Cancer Res. 2015;75:1169–1176. doi: 10.1158/0008-5472.CAN-14-3216. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Xu R., Bondreau A., Bissell M.J. Tissue architecture and function: Dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009;28:167–176. doi: 10.1007/s10555-008-9178-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Langmuir I. The constitutional and fundamental properties of solids and liquids. II. Liquids. J. Am. Chem. Soc. 1917;39:1848–1906. doi: 10.1021/ja02254a006. [ DOI ] [ Google Scholar ]
- 6. Singer S.J., Nicolson G.L. The Fluid Mosaic Model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Gorter E., Grendel F. On bimolecular layers of lipoids on the chromocytes of blood. J. Exp. Med. 1925;41:439–443. doi: 10.1084/jem.41.4.439. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Fricke H. The electrical capacity of cell suspensions. Phys. Rev. Series II. 1923;21:708–709. [ Google Scholar ]
- 9. Edidin M. Lipids on the frontier: A quarter century of cell-membrane bilayers. Nat. Rev. Mol. Cell Biol. 2003;4:414–418. doi: 10.1038/nrm1102. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Danielli J.F., Davson H. A contribution to the theory of permeability of thin films. J. Cell. Physiol. 1935;5:495–508. doi: 10.1002/jcp.1030050409. [ DOI ] [ Google Scholar ]
- 11. Robertson J.D. The ultrastructure of cell membranes and their derivatives. Biochem. Soc. Symp. 1959;16:3–43. [ PubMed ] [ Google Scholar ]
- 12. Benson A.A. On the orientation of lipids in chloroplast and cell membranes. J. Am. Oil Chem. Soc. 1966;43:265–270. doi: 10.1007/BF02609671. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Singer S.J. The molecular organization of membranes. Annu. Rev. Biochem. 1974;43:805–833. doi: 10.1146/annurev.bi.43.070174.004105. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Cramer W.A., Engelman D.M., von Heijne G., Rees D.C. Forces involved in the assembly and stabilization of membrane proteins. FASEB J. 1992;6:3397–3402. doi: 10.1096/fasebj.6.15.1464373. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Nicolson G.L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over cell surface components. Biochim. Biophys. Acta. 1976;457:57–108. doi: 10.1016/0304-4157(76)90014-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Rothman J.E., Lenard J. Membrane asymmetry. Science. 1977;195:743–753. doi: 10.1126/science.402030. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Daleke D.L. Regulation of transbilayer plasma membrane phospholipid asymmetry. J. Lipid Res. 2003;44:233–242. doi: 10.1194/jlr.R200019-JLR200. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Zimmerberg J., Kozlov M.M. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Baumgart T., Capraro B.R., Zhu C., Das S.L. Theromodynamics and mechanics of membrane curvature generation and sensing by proteins and lipids. Annu. Rev. Phys. Chem. 2011;62:483–506. doi: 10.1146/annurev.physchem.012809.103450. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Kusumi A., Suzuki K.G.N., Kasai R.S., Ritchie K., Fujiwara T.K. Hierarchical mesoscale domain organization of the plasma membrane. Trends Biochem. Sci. 2011;36:604–615. doi: 10.1016/j.tibs.2011.08.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Kusumi A., Fujiwara T.K., Chadda R., Xie M., Tsunoyama T.A., Kalay Z., Kasai R.S., Suzuki K.G. Dynamic organizing principals of the plasma membrane that regulate signal transduction: Commemorating the fortieth anniversary of Singer and Nicolson’s fluid-mosaic model. Annu. Rev. Cell Dev. Biol. 2012;28:215–250. doi: 10.1146/annurev-cellbio-100809-151736. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Jacobson K., Sheets E.D., Simson R. Revisiting the fluid mosaic model of membranes. Science. 1995;268:1441–1442. doi: 10.1126/science.7770769. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Nicolson G.L. The Fluid–Mosaic Model of Membrane Structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta. 2014;1838:1451–1466. doi: 10.1016/j.bbamem.2013.10.019. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Vereb G., Szöllősi J., Matko J., Nagy P., Farkas T., Vígh L., Mátyus L., Waldmann T.A., Damjanovich S. Dynamic, yet structured: The cell membrane three decades after the Singer-Nicolson model. Proc. Natl. Acad. Sci. USA. 2003;100:8053–8058. doi: 10.1073/pnas.1332550100. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Bernardino de la Serna J., Schütz G.J., Eggeling C., Cebecauer M. There is no simple model of the plasma membrane organization. Front. Cell Develop. Biol. 2016;4:106. doi: 10.3389/fcell.2016.00106. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Nicolson G.L., Masouredis S.P., Singer S.J. Quantitative two-dimensional ultrastructural distribution of Rho(D) antigenic sites on human erythrocyte membranes. Proc. Nat. Acad. Sci. USA. 1971;68:1416–1420. doi: 10.1073/pnas.68.7.1416. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Nicolson G.L., Hyman R., Singer S.J. The two-dimensional topographic distribution of H-2 histocompatibility alloantigens on mouse red blood cell membranes. J. Cell Biol. 1971;50:905–910. doi: 10.1083/jcb.50.3.905. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Garcia-Parajo M.F., Cambi A., Torreno-Pina J.A., Thompson N., Jacobson K. Nanoclustering as a dominant feature of plasma membrane organization. J. Cell Sci. 2014;127:4995–5005. doi: 10.1242/jcs.146340. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Ma Y., Pandzic E., Nicovich P.R., Yamamoto Y., Kwiatek J., Pageon S.V., Benda A., Rossy J., Gaus K. An intermolecular FRET sensor detects the dynamics of T cell receptor clustering. Nat. Commun. 2017;8:15100. doi: 10.1038/ncomms15100. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Pageon S.V., Tabarin T., Yamamoto Y., Ma Y., Nicovich P.R., Bridgeman J.S., Cohnen A., Benzing C., Gao Y., Crowther M.D., et al. Functional role of T-cell receptor nanoclusters in signal initiation and antigen discrimination. Proc. Nat. Acad. Sci. USA. 2016;113:e5454–e5463. doi: 10.1073/pnas.1607436113. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Goyette J., Gaus K. Mechanisms of protein nanoscale clustering. Curr. Opin. Cell Biol. 2017;44:86–92. doi: 10.1016/j.ceb.2016.09.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Geiger B., Yehuda-Levenberg S., Bershadsky A.D. Molecular interactions in the submembrane plaque of cell-cell and cell-matrix adhesions. Acta Anat. 1995;154:42–62. doi: 10.1159/000147751. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Geiger B., Bershadsky A.D., Pankov R., Yamada K.M. Transmembrane extracellular matrix-cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Goñi F.M. The basic structure and dynamics of cell membranes: An update of the Singer-Nicolson model. Biochim. Biophys. Acta. 2014;1838:1467–1476. doi: 10.1016/j.bbamem.2014.01.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Chichili C.R., Rogers W. Cytoskeleton-membrane interactions in membrane raft structure. Cell Mol. Life Sci. 2009;66:2319–2328. doi: 10.1007/s00018-009-0022-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Damjanovich S., Gáspár R., Jr., Pieri C. Dynamic receptor superstructures at the plasma membrane. Q. Rev. Biophys. 1997;30:67–106. doi: 10.1017/S0033583596003307. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Geiger B., Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr. Opin. Cell Biol. 2001;13:584–592. doi: 10.1016/S0955-0674(00)00255-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Schwarz U.S., Gardel M.L. United we stand: Integrating the actin cytoskeleton and cell-matrix adhesions in cellular mechanotransduction. J. Cell Sci. 2012;125:3051–3060. doi: 10.1242/jcs.093716. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Salas P.J., Vega-Salas D.E., Hochman J., Rodriguez-Boulan E., Edidin M. Selective anchoring in the specific plasma membrane domain: A role in epithelial cell polarity. J. Cell Biol. 1988;107:2363–2376. doi: 10.1083/jcb.107.6.2363. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Sezgin E., Levental I., Mayor S., Eggeling S. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017;18:361–374. doi: 10.1038/nrm.2017.16. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Janmey P.A., Lindberg U. Cytoskeletal regulation: Rich in lipids. Nat. Rev. Mol. Cell Biol. 2004;5:658–666. doi: 10.1038/nrm1434. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Simmons K., Gerl M.J. Revitalizing membrane rafts: New tools and insights. Nat. Rev. Mol. Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Simmons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Simmons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Simons K., Sampaio J.L. Membrane organization and lipid rafts. Cold Spring Harbor Perspect. Biol. 2010;3:a004697. doi: 10.1101/cshperspect.a004697. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Paulick M.G., Bertozzi C.R. The glycosylphosphatidylinositol anchor: A complex membrane anchoring structure for proteins. Biochemistry. 2008;47:6991–7000. doi: 10.1021/bi8006324. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Saha S., Anilkumar A.A., Mayor S. GPI-anchored protein organization and dynamics at the cell surface. J. Lipid Res. 2016;57:159–175. doi: 10.1194/jlr.R062885. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Sheets E.D., Lee G.M., Simson R., Jacobson K. Transient confinement of glycosylphosphatidylinositol-anchored protein in the plasma membrane. Biochemistry. 1997;36:12449–12458. doi: 10.1021/bi9710939. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Gowrishankar K., Ghosh S., Saha S., Rumamol C., Mayor S., Rao M. Active remodeling of cortical actin regulates spatiotemporal organization of cell surface molecules. Cell. 2012;149:1353–1367. doi: 10.1016/j.cell.2012.05.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Saha S., Lee I.H., Polley A., Groves J.T., Rao M., Mayor S. Diffusion of GPI-anchored proteins is influenced by the activity of dynamic cortical actin. Mol. Biol. Cell. 2015;26:4033–4045. doi: 10.1091/mbc.E15-06-0397. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Kalappurakkal J.M., Anikumar A.A., Patra C., van Zanten T.S., Sheetz M.P., Mayor S. Integrin mechano-chemical signaling generates plasma membrane nanodomains that promote cell spreading. Cell. 2019;177:1738–1756. doi: 10.1016/j.cell.2019.04.037. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Mouritsen O.G., Bloom M. Mattress model of lipid-protein interactions in membranes. Biophys. J. 1984;46:141–153. doi: 10.1016/S0006-3495(84)84007-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Mouritsen O.G. Model answers to lipid membrane questions. Cold Spring Harbor Perspect. Biol. 2011;3:a004622. doi: 10.1101/cshperspect.a004622. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Lindblom G., Orädd G. Lipid lateral diffusion and membrane heterogeneity. Biochim. Biophys. Acta. 2009;1788:234–244. doi: 10.1016/j.bbamem.2008.08.016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Sych T., Gurdap C.O., Wiedemann L., Sezgin E. How does liquid-liquid phase separation in model membrane reflect cell membrane heterogeneity? Membranes. 2021;11:323. doi: 10.3390/membranes11050323. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Yeagle P.L. Lipid regulation of cell membrane structure and function. FASEB J. 1989;3:1833–1842. doi: 10.1096/fasebj.3.7.2469614. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Ramstedt B., Slotte J.P. Sphingolipids and the formation of sterol-enriched ordered membrane domains. Biochim. Biophys. Acta. 2006;1758:1945–1956. doi: 10.1016/j.bbamem.2006.05.020. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Quinn P.J., Wolf C. The liquid-ordered phase in membranes. Biochim. Biophys. Acta. 2009;1788:33–46. doi: 10.1016/j.bbamem.2008.08.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Pike L.J. Rafts defined: A report on the Keystone Symposium on Lipid Rafts and cell function. J. Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Neumann A.K., Itano M.S., Jacobson K. Understanding lipid rafts and other related membrane domains. F1000 Biol. Rep. 2010;2:31–36. doi: 10.3410/B2-31. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Rossy J., Ma Y., Gaus K. The organization of the cell membrane: Do proteins rule lipids? Curr. Opin. Chem. Biol. 2014;20:54–59. doi: 10.1016/j.cbpa.2014.04.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Bagatolli L.A., Ipsen J.H., Simonsen A.C., Mouritsen O.G. An outlook on the organization of lipids in membranes: Searching for a realistic connection with the organization of biological membranes. Prog. Lipid Res. 2010;49:378–389. doi: 10.1016/j.plipres.2010.05.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Mouritsen O.G. Lipids, curvature and nano-medicine. Eur. J. Lipid Sci. Technol. 2011;113:1174–1187. doi: 10.1002/ejlt.201100050. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Jacobson K., Liu P., Lanerholm B.C. The lateral organization and mobility of plasma membrane components. Cell. 2019;177:806–819. doi: 10.1016/j.cell.2019.04.018. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Shurer C.R., Kuo J.C.-H., Roberts L.M., Gandhi J.G., Colville M.J., Enoki T.A., Pan H., Su J., Boble J.M., Hollander M.J., et al. Physical principles of membrane shape regulation by the glycocalyx. Cell. 2019;177:1757–1770. doi: 10.1016/j.cell.2019.04.017. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Kuo J.C.-H., Paszek M.J. Glycocalyx curving the membrane: Forces emerging from the cell exterior. Annu. Rev. Cell Dev. Biol. 2021;37:257–283. doi: 10.1146/annurev-cellbio-120219-054401. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Kusumi A., Sako Y., Yamamoto M. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys. J. 1993;65:2021–2040. doi: 10.1016/S0006-3495(93)81253-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 68. Lev S. Non-vescular lipid transport by lipid-transfer proteins and beyond. Nat. Rev. Mol. Cell Biol. 2010;11:729–750. doi: 10.1038/nrm2971. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Toulmay A., Prinz W.A. Lipid transfer and signaling at organelle contact sites: The tip of the iceberg. Curr. Opin. Cell Biol. 2011;23:458–463. doi: 10.1016/j.ceb.2011.04.006. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Diaz-Rohrer B., Levental K.R., Levental I. Rafting through traffic: Membrane domains in cellular logistics. Biochim. Biophys. Acta. 2014;1838:3003–3013. doi: 10.1016/j.bbamem.2014.07.029. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Andrews N.W., Almeida P.E., Corrotte M. Damage control: Cellular mechanisms of plasma membrane repair. Trends Cell Biol. 2014;24:734–742. doi: 10.1016/j.tcb.2014.07.008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Leabu M., Nicolson G.L. Cell secretion and membrane fusion: Highly significant phenomena in the life of a cell. Discoveries. 2014;2:e30. doi: 10.15190/d.2014.22. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. White J.M. Membrane fusion. Science. 1992;258:917–924. doi: 10.1126/science.1439803. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Lu X., Kang Y.J.C. Cell fusion as a hidden force in tumor progression. Cancer Res. 2009;69:8536–8539. doi: 10.1158/0008-5472.CAN-09-2159. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Floyd D.L., Ragains J.R., Skehel J.J., Harrison S.C., van Oijen A.M. Single particle kinetics of influenza virus membrane fusion. Proc. Nat. Acad. Sci. USA. 2008;105:15382–15387. doi: 10.1073/pnas.0807771105. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Leabu M. Membrane fusion in cells: Molecular machinery and mechanisms. J. Cell Mol. Med. 2006;10:423–427. doi: 10.1111/j.1582-4934.2006.tb00409.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. McMaster C.R. Lipid metabolism and vesicle trafficking: More than just greasing the transport machinery. Biochem. Cell Biol. 2001;79:681–692. doi: 10.1139/o01-139. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Tahir M.A., Guven Z.P., Arriaga L.R., Tinao B., Yang Y.S., Bekdemir A., Martin J.T., Bhanji A.N., Irvine D., Stellacci F., et al. Calcium-triggered fusion of lipid membranes is enabled by amphiphilic nanoparticles. Proc. Nat. Acad. Sci. USA. 2020;117:18470–18476. doi: 10.1073/pnas.1902597117. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Chernomordik L.V., Kozlov M.M. Mechanics of membrane fusion. Nat. Struct. Mol. Biol. 2008;15:675–683. doi: 10.1038/nsmb.1455. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Holthuis J.C., van Meer G., Huitema K. Lipid microdomains, lipid translocation and the organization of intracellular membrane transport. Mol. Membr. Biol. 2003;20:231–241. doi: 10.1080/0988768031000100768. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Jena B.P. Discovery of the Porosome: Revealing the molecular mechanism of secretion and membrane fusion in cells. J. Cell Mol. Med. 2004;8:1–21. doi: 10.1111/j.1582-4934.2004.tb00255.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Mayor S., Presley J.F., Maxfield F.R. Sorting of membrane components from endosomes and sub sequent recycling to the cell surface occurs by a bulk flow process. J. Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Cocucci E., Racchetti G., Meldolesi J. Shedding microvesicles: Artifacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Beaudoin A.R., Grondin G. Shedding of vesicular material from the cell surface of eukaryotic cells: Different cellular phenomenon. Biochim. Biophys. Acta. 1991;1071:203–219. doi: 10.1016/0304-4157(91)90014-N. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Turturici G., Tinnirello R., Sconzo G., Gerael F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: Advantages and disadvantages. Am. J. Physiol. Cell Physiol. 2014;306:C621–C633. doi: 10.1152/ajpcell.00228.2013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Ratajczak J., Wysocznski M., Hayek F., Janowska-Wieczorek A., Ratajczak M.Z. Membrane-derived microvessels: Important and under-appreciated mediators of cell-to-cell communication. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Martins V.R., Dias M.S., Hainaut P. Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr. Opin. Oncol. 2013;25:66–79. doi: 10.1097/CCO.0b013e32835b7c81. [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Maheshwari S., Singh A.K., Arya R.K., Pandey D., Singh A., Datta D. Exosomes: Emerging players of intercellular communication in tumor microenvironment. Discoveries. 2014;2:e26. doi: 10.15190/d.2014.18. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Hoshino D., Kirkbride K.C., Costello K., Clark E.S., Sinha S., Grega-Larson N., Tyska M.J., Weaver A.M. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;12:1159–1168. doi: 10.1016/j.celrep.2013.10.050. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 90. Nicolson G.L., Ash M.E. Membrane Lipid Replacement for chronic illnesses, aging and cancer using oral glycerolphospholipid formulations with fructooligosaccharides to restore phospholipid function in cellular membranes, organelles, cells and tissues. Biochim. Biophys. Acta Biomembr. 2017;1859:1704–1724. doi: 10.1016/j.bbamem.2017.04.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Küllenberg D., Taylor L.A., Schneider M., Massing U. Health effects of dietary phospholipids. Lipids Health Dis. 2012;11:e3. doi: 10.1186/1476-511X-11-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 92. Nicolson G.L. Membrane Lipid Replacement: Clinical studies using a natural medicine approach to restoring membrane function and improving health. Intern. J. Clin. Med. 2016;7:133–143. doi: 10.4236/ijcm.2016.72015. [ DOI ] [ Google Scholar ]
- 93. Nicolson G.L., Rosenblatt S., Ferreira de Mattos G., Settineri R., Breeding P., Ellithorpe R.R. Clinical uses of Membrane Lipid Replacement supplements in restoring membrane function and reducing fatigue in chronic diseases and cancer. Discoveries. 2016;4:e54. doi: 10.15190/d.2016.1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. Gundermann K.J. The “Essential” Phospholipids as a Membrane Therapeutic. European Society of Biochemical Pharmacology; Szcecin, Poland: 1993. [ Google Scholar ]
- 95. Zierenberg O., Grundy S.M. Intestinal absorption of polyenephosphatidylcholine in man. J. Lipid Res. 1982;23:1136–1142. doi: 10.1016/S0022-2275(20)38050-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Nicolson G.L., Breeding P.C., Settineri R., Ferreira de Mattos G. Aging and chronic illnesses: Membrane Lipid Replacement for restoring mitochondrial function and reducing fatigue, pain, and other symptoms in aged individuals. [(accessed on 1 November 2021)];Bioact. Comp. Health Dis. 2020 3:194–203. doi: 10.31989/bchd.v3i10.749. Available online: https://ffhdj.com/index.php/BioactiveCompounds/article/view/749/1339 . [ DOI ] [ Google Scholar ]
- 97. Kroenke K., Wood D.R., Mangelsdorff A.D., Meiero N.J., Powell J.B. Chronic fatigue in primary care. Prevalence, patient characteristics, and outcome. J. Am. Med. Assoc. 1988;260:929–934. doi: 10.1001/jama.1988.03410070057028. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Filler K., Lyon D., Bennett J., McCain N., Elswick R., Lukkahatai N., Saligan L.N. Association of mitochondrial dysfunction and fatigue: A review of the literature. BBA Clin. 2014;1:12–23. doi: 10.1016/j.bbacli.2014.04.001. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 99. Nicolson G.L. Mitochondrial dysfunction and chronic disease: Treatment with natural supplements. [(accessed on 1 November 2021)];Integr. Med. 2014 13:35–43. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4566449 . [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 100. Komaroff A.L., Buchwald D. Symptoms and signs of chronic fatigue. Rev. Infect. Dis. 1991;13((Suppl. S1)):S8–S11. doi: 10.1093/clinids/13.Supplement_1.S8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Agadjanyan M., Vasilevko V., Ghochikyan A., Berns P., Kesslak P., Settineri R.A., Nicolson G.L. Nutritional supplement (NTFactor®) restores mitochondrial function and reduces moderately severe fatigue in aged subjects. J. Chronic Fatigue Syndr. 2003;11:23–36. doi: 10.1300/J092v11n03_03. [ DOI ] [ Google Scholar ]
- 102. Nicolson G.L. Lipid replacement therapy: A nutraceutical approach for reducing cancer-associated fatigue and the adverse effects of cancer therapy while restoring mitochondrial function. Cancer Metastasis Rev. 2010;29:543–552. doi: 10.1007/s10555-010-9245-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Nicolson G.L., Settineri R., Ellithorpe E. Lipid Replacement Therapy with a glycophospholipid formulation with NADH and CoQ10 significantly reduces fatigue in intractable chronic fatiguing illnesses and chronic Lyme disease. Int. J. Clin. Med. 2012;3:164–170. doi: 10.4236/ijcm.2012.33034. [ DOI ] [ Google Scholar ]
- 104. Nicolson G.L., Settineri R., Ellithorpe E. Glycophospholipid formulation with NADH and CoQ10 significantly reduces intractable fatigue in chronic Lyme disease patients: Preliminary report. [(accessed on 1 November 2021)];Funct. Food Health Dis. 2012 2:35–47. doi: 10.31989/ffhd.v2i3.100. Available online: http://www.functionalfoodscenter.net/files/50313126.pdf . [ DOI ] [ Google Scholar ]
- 105. Nicolson G.L., Settineri R., Ferreira G., Breeding P. Reduction of pain, fatigue, gastrointestinal and other symptoms and improvement in quality of life indicators in fibromyalgia patients with Membrane Lipid Replacement glycerolphospholipids and controlled-release caffeine. Intern. J. Clin. Med. 2018;9:560–579. doi: 10.4236/ijcm.2018.97051. [ DOI ] [ Google Scholar ]
- 106. Nicolson G.L., Breeding P.C. Membrane Lipid Replacement for reduction of pain, fatigue, gastrointestinal and other symptoms in patients with peripheral pain: Case reports. [(accessed on 1 November 2021)];Case Rep. Rev. 2020 1:1–3. doi: 10.33425/2693-1516.1007. Available online: https://www.sciencexcel.com/articles/Membrane%20Lipid%20Replacement%20for%20reduction%20of%20pain%20%20fatigue%20%20gastrointestinal%20and%20other%20symptoms%20in%20patients%20with%20peripheral%20pain%20%20Case%20reports . [ DOI ] [ Google Scholar ]
- 107. Woolf C.J. What is this thing called pain? J. Clin. Investig. 2010;120:3742–3744. doi: 10.1172/JCI45178. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 108. Porter J., Turk D.C. Management of pain: Best of times, worst of times. Clin. J. Pain. 2001;17:107–109. doi: 10.1097/00002508-200106000-00001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 109. Sikandar S., Dickenson A.H. Visceral pain: The ins and outs, the ups and downs. Curr. Opin. Support. Palliat. Care. 2012;6:17–26. doi: 10.1097/SPC.0b013e32834f6ec9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 110. Vandewauw I., De Clercq K., Mulier M., Held K., Pinto S., Van Ranst N., Segal A., Voet T., Vennekens R., Zimmermann K., et al. A TRP channel trio mediates acute noxious heat sensing. Nature. 2018;555:662–666. doi: 10.1038/nature26137. [ DOI ] [ PubMed ] [ Google Scholar ]
- 111. Monteiro de Araujo D.S., Nassini R., Geppetti P., De Logu F. TRPA1 as a therapeutic target for nociceptive pain. Expert Opin. Therapeut. Targ. 2020;24:997–1008. doi: 10.1080/14728222.2020.1815191. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 112. Clapham D.E., Bunnels L.W., Strübing C. The TRP ion channel family. Nat. Rev. Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Mickle A.D., Shepherd A.J., Mohapatra D.P. Nociceptive TRP channels: Sensory detectors and transducers in multiple pain pathologies. Pharmaceuticals. 2016;9:72. doi: 10.3390/ph9040072. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 114. Ferreira G., Raddatz N., Lorenzo Y., González C., Latorre R. Biophysical and molecular features of thermosensitive TRP channels involved in sensory transduction. In: Madrid R., Bacigalupo J., editors. TRP Channels in Sensory Transduction. Springer; Cham, Switzerland: 2015. pp. 1–39. [ DOI ] [ Google Scholar ]
- 115. Hilgemann D.W., Ball R. Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [ DOI ] [ PubMed ] [ Google Scholar ]
- 116. Rohacs T. Phosphoinositide regulation of TRP channels. Handb. Exp. Pharmacol. 2014;223:1143–1176. doi: 10.1007/978-3-319-05161-1_18. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 117. Lukacs V., Thyaggarajan B., Varnai P., Balla A., Balla T., Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J. Neurosci. 2007;27:7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 118. Fan Z., Makielski J.C. Anionic phospholipids activate ATP-sensitive potassium channels. J. Biol. Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Hansen S.B., Tao X., MacKinnon R. Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature. 2011;477:495–498. doi: 10.1038/nature10370. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
- View on publisher site
- PDF (1.0 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Fluid mosaic model
Fluid mosaic model n., plural: [fluːɪd məʊˈzeɪk mɒdl̩] Definition: Model describing the structure of the plasma membrane as a dynamic and fluid arrangement of lipids, proteins, and carbohydrates
Table of Contents
Fluid Mosaic Model Definition
What is the fluid mosaic model? The fluid mosaic model is a three-dimensional representation of the structure and dynamics of the plasma membrane proposed by S.J. Singer and G.L. Nicolson in 1972. The fluid mosaic model describes the plasma membrane as a ‘fluid` and a ‘mosaic’ structure. According to this model, the plasma membrane is a phospholipid bilayer structure composed of fluid and a mosaic of diverse molecules, which is essential in understanding how the plasma membrane functions. The fluidity of such a biological membrane refers to its characteristic dynamicity. This means that the plasma membrane structure is dynamic rather than a rigid structure of lipids, proteins, and carbohydrates. The plasma membrane components have the ability to move within the plane of the membrane. There are other models but the fluid mosaic model is the widely accepted representation of the biological membranes to this day.
Let us learn more about the fluid mosaic model and find the answers to common questions like ‘What does the fluid mosaic model state? What is the cell membrane made of? How do the cell membrane components affect the general cell membrane functions?’ W e can start with the fluid mosaic model definition in biology and what it states:
Biology definition: A fluid mosaic model of the cell membrane (or plasma membrane) is a conceptual framework for its structure and behavior. This model states that the cell membrane is composed of a fluid lipid bilayer with proteins that are embedded within it. It emphasizes the fluid nature of the structure wherein the lipids and proteins are capable of lateral movement within the membrane as well as its mosaic nature as it contains a variety of proteins and lipids, as well as certain carbohydrates, that are distributed unevenly. This model acknowledges that the membrane is selectively permeable, allowing certain molecules to pass through while restricting the others. The selective permeability feature of the cell membrane is crucial to its role in cellular homeostasis by regulating the entry and exit of substances between the cell and its environment.
Development Of The Fluid Mosaic Model
Prior to the conception of the fluid mosaic model, the prevailing notion is that the cell membranes were static and rigid structures. This perception changed with the advancing studies on biological membranes using newer technologies, especially in microscopy techniques.
The development of the fluid mosaic model represented a significant paradigm shift in our understanding of cellular membranes. It transformed our perception of membranes from rigid barriers to dynamic entities, laying the foundation for further investigations into membrane biology and its implications in various cellular processes.
With the emergence of microscopes, the presence of cells as the fundamental unit of life has been recognized in the 1600s. Our understanding of the cells from then on expanded into identifying cellular parts, such as the cell membrane that was recognized in the early 1800s. Its permeability depending on conditions has also long been recognized.(Kalkan & Esrefoglu, 2020) (Hücre Membranının Keşfi: Tarihsel Bir Bakış, 2019) The chemical components of the cell membrane (i.e. lipids and proteins) were then identified in 1915.
How our understanding of the cell membrane was shaped? What are the key events that led to the development of the fluid mosaic model of the cell membranes? Let’s find out.
- » 1890s-Early 1900s: Charles Ernest Overton proposed the so-called Overton Biomembrane Model where he proposed biomembranes being made up of lipids based on his observation wherein lipid-soluble substances were transported across biomembranes. In 1904, Nathanson proposed a mosaic membrane theory , suggesting that the membrane contained mosaic domains to account for the different pathways for the entry of soluble or insoluble materials. (Hücre Membranının Keşfi: Tarihsel Bir Bakış, 2019)
- » 1920s: Evert Gorter and François Grendel proposed the lipid bilayer model in 1925, suggesting that the cell membrane consists of a double layer of lipids where the hydrophobic heads face outwards while the hydrophobic tails face inwards. Although incomplete, their model provided the basic framework of the cell membrane.
- » 1930s: The thought that the membrane was surrounded by proteins is suggested by Harvey and Kennet S. Cole in 1932. Then, in 1935, James Frederic Danielli and Hugh Davson Fricke proposed the sandwich membrane model (Davson-Danielli model or protein-protein model) in which a lipoid center is sandwiched between protein layers (i.e. protein-lipid-protein) . Their work recognized the importance of proteins, particularly their role in various processes of biological membranes.
- » 1950s: Cell biologists were able to view cell membranes in significantly improved resolutions via electron microscopy. Membranes were seen as three layers. J. David Robertson came up with the unit membrane model where he theorized (1) that all cell membranes had a generalized tri-laminar structure (which corresponds to Danielli and Davson’s protein-lipid-protein arrangement of the cell membrane) and (2) that they perform a similar function.
- » 1960s: in 1964, Brady RO and Trams EG’s works laid important foundations for Singer and Nicolson’s model, such as the proteins entering the lipid matrix layer indicating that the membrane is fluid. (Kalkan & Esrefoglu, 2020)
- » 1970s: Seymour Jonathan Singer and Garth L. Nicolson proposed in 1972 the fluid mosaic model in their paper, “ The Fluid Mosaic Model of the Structure of Cell Membranes ” published in Science. Here, they introduced the fluidity of the cell membrane and its being composed of a mosaic of lipids and proteins.
Over time, further research has been conducted that helped expand the understanding of the fluid mosaic model, refining and enhancing our understanding of biological membranes.
Various glycobiology researchers have contributed to the understanding of the glycocalyx and the role of carbohydrates in the cell membrane, which led to refinements of the fluid mosaic model by incorporating the spatial structure and role of carbohydrates, such as glycolipids and glycoproteins in the cell membrane.
Advances in microscopy in 1977 led to the visualization of the internal structure of the cell membrane, supporting the fluid mosaic model. Further advances in molecular biology and protein purification techniques in the ’80s and ’90s, in turn, aided in the characterization of integral membrane proteins, providing concrete evidence of the presence of proteins in the cell membrane. One of them is the joint work of Kai Simons and Elina Ikonen who published the article “Functional rafts in cell membranes” in Nature in 1997 . They discussed the concept of “lipid rafts” , the cholesterol-rich microdomains in the cell membrane.
Through the work of scientists and researchers, the development of the fluid mosaic model has proven to be a significant milestone in cell biology as it paved the way to other important milestones, especially in the field of drug development and treatments.
Watch this vid about the fluid mosaic model:
Functions And Components Of Biological Membranes
Singer and Nicolson’s Fluid Mosaic Model provided several key insights into the features that help elucidate the components and functions of biological membranes. Some of them are as follows:
- Selective permeability. Certain molecules can diffuse unaided through the membrane easily (while preventing most substances to pass through).
- Membrane protein functions. The proteins can also allow movement within the lipid bilayer. With proteins moving laterally, it allows biological membranes to carry out processes, such as protein-protein interactions and signal transductions.
- Vesicle Formation and Fusion. Due to membrane flexibility, it allows the membrane to undergo curvature and thus, create vesicles for the transport of substances within and outside the cell.
- Cellular homeostasis. Because the membrane has the ability to alter its shape and remodel, it can therefore repair itself by fusing to maintain its structural integrity, which is essential for cellular homeostasis.
- Selective permeability. While some small, non-polar molecules (e.g., oxygen and carbon dioxide) can diffuse freely through the lipid bilayer due to the membrane’s flexibility and fluidity, larger molecules and ions cannot. However, they can still pass through albeit not as easily. Their transport will be regulated via specialized transport proteins before they can be allowed to cross the membrane.
- Efficient cell signaling. The asymmetry in the cell membrane enables one side of the membrane to be concentrated with signaling molecules. This is crucial in certain events, such as in the detection of external signals and leading to the initiation of the appropriate response.
- Specialized function. Because membranes would vary in spatial arrangements and compositions, the function of the cell would also therefore vary. Their cell membrane will be able to carry out special functions, depending on the cellular needs and environmental changes.
- Efficient trafficking. The asymmetrical distribution of proteins in a biological membrane is crucial to various biological processes, such as endocytosis. For instance, clathrin-coated vesicles formed during receptor-mediated endocytosis exhibit membrane asymmetry. The cytoplasmic face of the vesicle membrane contains proteins such as clathrin and adaptins that mediate the vesicle formation. The luminal face (i.e. facing the extracellular matrix or space), in turn, displays receptor proteins that have undergone internalization. Not only is this crucial in cell membranes. Asymmetry is also important in the plasma membranes of the mitochondria. In particular, the inner mitochondrial membrane (which is the site of the electron transport chain of cellular respiration) is also the site of ATP synthases, which pump H + ions into the mitochondrial lumen, for ATP production.
- Firstly, such a structural and physiological organization enables the cell membrane to efficiently serve as a protective covering against pathogens and harmful substances.
- Secondly, it enables compartmentalization. Plasma membranes create distinct compartments within cells, enabling efficient organization and specialization of cellular processes.
How the cell membranes (plasma membranes) are able to possess these characteristics is due to the membrane structures that made them. Here are the fundamental biological membrane structures: The presence of unsaturated fatty acids introduces kinks in the hydrocarbon chains, which contributes to the membrane’s fluidity.
Phospholipids
In Figure 2, the model shows a membrane bilayer of phospholipid molecules that make up the bulk of the structure. In Figure 5 below, take note of the phosphoglyceride (the primary type of membrane phospholipids). It is made up of two parts: the head , which is made up of phosphate and glycerol molecules, and the tail , which is made up of saturated and unsaturated fatty acids . Anther membrane phospholipids are sphingolipids (have a sphingosine backbone rather than a glycerol backbone).
The phospholipids are amphipathic molecules. This means they have both water-loving ( hydrophilic ) and water-fearing ( hydrophobic ) regions. The hydrophobic core forms from the hydrophobic interactions of the tails resulting in a bilipid layer orientation wherein the hydrophilic heads face toward the aqueous environment.
These phospholipids are capable of moving laterally within the plane of the membrane. They can also move transversely (referred to as flip-flop ) although they do so infrequently and with the aid of enzymes called flippases.
The phospholipids provide a means by which the membrane proteins can be inserted or embedded, creating membrane domains (specialized regions within the membrane composed of lipids and protein complexes). They are also capable of interacting on the cell membrane surface with other molecules, such as carbohydrates and other proteins for cell signaling and communication.
Singer and Nicoson highlighted two types of proteins in cell membranes:
- Peripheral membrane proteins: peripheral proteins are proteins present or associated with the membrane surface (i.e., cell surface facing the inside or the outside of the cell), hence, the name “peripheral” ). See Figure 1.
- Integral membrane proteins: integral proteins are cell membrane proteins that notably contribute to the structure of the membrane. Their hydrophobic region interacts with the hydrophobic core of the lipid bilayer, thus, are able to anchor themselves in place. Because they are fairly embedded they are sometimes described or referred to as intrinsic proteins . They span the lipid bilayer partially or completely. For instance, transmembrane proteins span the entire width of the lipid bilayer, extending into both the cytoplasm and the extracellular fluid. See Figure 1.
Carbohydrates
Figure 1 shows carbohydrates protruding on the outer cell surfaces. Carbohydrates when attached to membrane lipids form glycolipids . Other carbohydrates attach to proteins, forming glycoproteins . The carbohydrate chains extend outward into the extracellular space. A layer of glycolipids or glycoproteins forms glycocalyx .
An example of glycoproteins on the membrane surface is the major histocompatibility complex (MHC) protein, which is a group of glycoproteins in the cell membranes of nucleated cells in humans and other vertebrates. Another is the glycosyltransferases , which when functional, can add specific sugars to H antigen to form A antigen and/or B antigen . Together, they are transported to the cell surface where the glycosyltransferases eventually become part of the cell membrane akin to the integral membrane proteins and display the antigen sugars on the surface of the red blood cells.
Did you know that the ABO blood type test is based on certain carbohydrate antigens?
The carbohydrate antigens of red blood cell membranes are used as a basis for blood typing tests. In particular, the test is based on the presence or absence of the ABO blood group system , which is a system of specific carbohydrate molecules that act as antigens on the surface of red blood cells. These antigens are A , B , and H antigens . Here is a chart to explain how the different blood types are determined based on the antigens present in the RBCs.
Cholesterol
Although the seminal paper of Singer and Nicolson did not specifically mention cholesterol, subsequent studies have eventually recognized cholesterol as part of the lipid composition of the cell membrane.
(While the traditional view of cholesterol is primarily associated with animal cell membranes only, there are some interesting discoveries of recent implicating that cholesterol molecules may occur as well in certain types of cells of other groups of living things, such as bacteria and plants, which have only phytosterols or cholesterol-like compounds. If so, cholesterol would still rather be more common in animal cells.)
Remember that animal cells lack a cell wall , and therefore, the presence of cholesterol would help in providing structural support. Cholesterol is essential in maintaining the structural fluidity, integrity, and functionality of cell membranes.
How does cholesterol affect membrane fluidity? What is the function of cholesterol in the cell membrane? Cholesterol is an amphipathic lipid molecule embedded within the phospholipid core. It acts as a “fluidity buffer” by reducing the fluidity of the membrane at high temperatures and increasing it at low temperatures .
At high temperatures, cholesterol restrains the movement of phospholipids, reducing their mobility and preventing excessive fluidity. At low temperatures, cholesterol prevents the close packing of phospholipids, increasing their mobility and maintaining fluidity.
Apart from fluidity, cholesterol also plays a role in the formation of lipid rafts serving as platforms for various cellular processes, including signal transduction and membrane trafficking.
Other Models For Membrane Structure
There have been other models proposed to describe membrane structure. Nevertheless, the fluid mosaic model remains widely accepted.
For details, see Development Of The Fluid Mosaic Model .
Apart from the ones mentioned above, here are other models that have provided additional insights and perspectives on the organization and behavior of cell membranes:
- Lipid Raft Model: proposes that the cell membrane is organized into distinct microdomains called lipid rafts, enriched in specific lipids and associated proteins.
- Protein-Induced Bilayer Deformation Model: suggests that proteins act as scaffolds that can induce local deformations in the lipid bilayer structure, leading to changes in membrane curvature and organization.
Choose the best answer.
Send Your Results (Optional)
Further reading, cell structure.
- Singer, S.J., & Nicolson, G.L. (1972). The fluid mosaic model of the structure of cell membranes. Science, 175(23), 720-731.
- Simons, K., & Ikonen, E. (1997). Functional rafts in cell membranes. Nature, 387(6633), 569-572.
- McMahon, H.T., & Gallop, J.L. (2005). Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature, 438(7068), 590-596.
- Molnar, C., & Gair, J. (2015, May 14). 3.4 The Cell Membrane. Opentextbc.ca; BCcampus. https://opentextbc.ca/biology/chapter/3-4-the-cell-membrane/
- Kübra Tuğçe Kalkan, & Mukaddes Esrefoglu. (2020). The Cell Membrane: A Historical Narration. 8(1), 81–88. https://doi.org/10.14235/bas.galenos.2019.3131
- Hücre Membranının Keşfi: Tarihsel Bir Bakış. (2019). Bezmialemscience.org. https://www.bezmialemscience.org/archives/archive-detail/article-preview/the-cell-membrane-a-historical-narration/34643#:~:text=In%201904%20Nathanson%20put%20forward,presipitation%20membrane%20(artificial%20membrane).
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.
Last updated on July 21st, 2023
You will also like...
Biological cell introduction, related articles....
No related articles found

- Why Does Water Expand When It Freezes
- Gold Foil Experiment
- Faraday Cage
- Oil Drop Experiment
- Magnetic Monopole
- Why Do Fireflies Light Up
- Types of Blood Cells With Their Structure, and Functions
- The Main Parts of a Plant With Their Functions
- Parts of a Flower With Their Structure and Functions
- Parts of a Leaf With Their Structure and Functions
- Why Does Ice Float on Water
- Why Does Oil Float on Water
- How Do Clouds Form
- What Causes Lightning
- How are Diamonds Made
- Types of Meteorites
- Types of Volcanoes
- Types of Rocks
Fluid Mosaic Model
The fluid mosaic model is a way biologists use to describe the structure of biological membranes, such as the cell membrane . It was first proposed by Seymour Jonathan Singer and Garth L. Nicolson in 1972. The model has been modified in parts over time, keeping the basic concept the same.
It describes the structure of the cell (plasma) membrane as a mosaic of components – proteins, phospholipids, carbohydrates, and cholesterol giving the membrane a fluid character. However, the proportion of the components varies with cell type. For example, the inner mitochondrial membrane contains 76% protein and 24% lipid, while myelin has 18% protein and 76% lipid in its membrane.
Fluid Mosaic Model and Cell Membrane
According to the fluid mosaic model, the main components of the cell membrane are:
- Phospholipids : The fabric of the membrane forms a bilayer with phosphate heads facing outwards and fatty acid tails facing inwards. They are amphiphilic or dual-loving.
- Cholesterol : A steroid that regulates the entry and exit of molecules in and out of the cell. It is attached to a single phospholipid and between the two phospholipid layers.
- Proteins : Based on the position in the membrane, they are of two types: integral and peripheral proteins.
- Carbohydrates : They are attached to proteins on the outside.
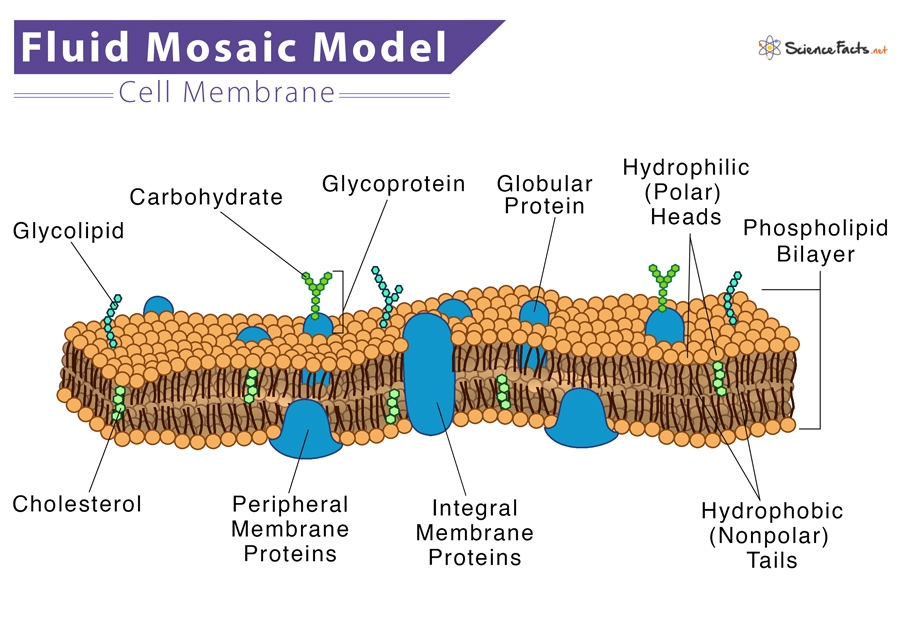
The model describes the structure of the cell membrane as a mosaic of these components having a thickness of 5-10 nm.
Phospholipids
Phospholipids are amphiphilic, having water-attraction (hydrophilic) and water-repulsion (hydrophobic) regions. The polar, hydrophilic head is constantly in contact with the fluid inside and outside the cell. In contrast, the non-polar, hydrophobic tail is away from the aqueous environment. Each phospholipid molecule has a glycerol backbone with two fatty-acid molecules and a phosphate group attached to it.
In an aqueous environment, the hydrophobic molecules arrange themselves to form a ball or a cluster. Hydrophilic regions form hydrogen bonds with water and other polar molecules. Thus the surface of the cell membrane facing the interior and exterior of the cell is hydrophilic. This arrangement allows phospholipid molecules to form a bilayer structure that separates the cell’s interior from the exterior.
Cholesterol
Cholesterol is found within the phospholipids, allowing the membrane to retain its permeability and integrity even if there is a temperature change. Cholesterol also prevents the compaction of the hydrophobic tails of lipids at low temperatures and the membrane’s expansion under heat. It helps the cell membrane selectively permeable to larger molecules while allowing small molecules like carbon dioxide and oxygen to pass through easily.
Proteins are the second major component of the cell membrane and are randomly arranged within the lipid bilayer. Integral proteins are entirely integrated into the membrane structure. Their hydrophobic membrane-spanning part interacts with the hydrophobic part of the bilayer.
Single-pass integral proteins have a single membrane-spanning region consisting of 20–25 amino acids. Some integral proteins span only part of the membrane. In contrast, others have a transmembrane region from one side to the other. In contrast, multi-pass proteins transverse the membrane multiple times that are folded and embedded in the membrane. Thus, this type of membrane protein has a hydrophilic region(s) and one or several mildly hydrophobic regions.
This arrangement of proteins tends to orient them along with the phospholipids, with the protein’s hydrophobic region adjacent to the phospholipid’s tails and the hydrophilic regions protruding from the membrane, having contact with the cytosol or the extracellular fluid.
Carbohydrates
Apart from the three main parts, a cell membrane contains carbohydrates on the exterior surface of cells attached to proteins (forming glycoproteins) or lipids (forming glycolipids). Each carbohydrate chain consists of 2-60 monosaccharide units. With peripheral proteins, carbohydrate chains form specialized sites that allow cell-cell recognition. These carbohydrates on the exterior cell surface in the glycoproteins and glycolipids are together called the glycocalyx. It is hydrophilic and attracts water to the cell surface and thus helps to absorb substances dissolved in water.
All these components are arranged to give it a mosaic pattern. Scanning electron microscope images show that the embedded molecules, such as proteins and lipids, can move sideways through the membrane, which means the membrane is fluid-like and not solid. Such movement causes a change in the mosaic pattern of the cell membrane. With the help of proteins, lipids, and carbohydrates, the cell constantly interacts with the outside environment, thus allowing the import of ions, hormones, and food inside the cell and exporting waste products.
What does Fluid Mosaic Model Do
The fluid mosaic model also helps to recognize the functions of the different components in the cell membrane. It also explains how the membrane functions as a barrier between the cell cytosol and the extracellular fluid and develop cell-cell communication.
- Fluid-Mosaic Model – Khanacademy.org
- Components and Structure – Fluid Mosaic Model – Bio.libretexts.org
- Fluid-Mosaic Model – Ib.bioninja.com.au
- The Fluid Mosaic Model of the Cell Membrane – Study.com
- The Fluid Mosaic Model – Advanced – Ck12.org
- The Fluid Mosaic Model of the Structure of Cell Membranes – Science.org
Article was last reviewed on Wednesday, February 1, 2023
Related articles
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Popular Articles

Join our Newsletter
Fill your E-mail Address

Related Worksheets
- Privacy Policy
© 2024 ( Science Facts ). All rights reserved. Reproduction in whole or in part without permission is prohibited.
Fluid Mosaic Model of Plasma Membrane
What is Fluid Mosaic Model of Plasma Membrane? – Fluid Mosaic Model Definition
Components of plasma membrane, the fluid mosaic model of plasma membrane, development of the fluid mosaic model, factors affecting fluidity of plasma membrane, fluid mosaic model function, what is the fluid mosaic model, who proposed the fluid mosaic model, what are the components of the fluid mosaic model, how do molecules move within the membrane, what is the function of cholesterol in the fluid mosaic model, what types of proteins are embedded in the membrane, how are proteins able to move within the membrane, what is the significance of the fluid mosaic model, what factors affect the fluidity of the membrane, how has the fluid mosaic model been supported by scientific evidence.
The fluid mosaic model is one method to comprehend biological membranes, consistent with the majority of experimental findings. According to this hypothesis, membrane components such as proteins and glycolipids form a mobile mosaic in the fluid-like environment formed by a sea of phospholipids. There are limitations on lateral movement, and subdomains within the membrane have distinct functions.
- Fluid Mosaic Model is a generally accepted model for describing the structure of the plasma membrane that encircles cells. In 1972, S. J. Singer and Garret L. Nicolson proposed the model, which has since become a fundamental paradigm in cell biology.
- The term “fluid” refers to the fact that the plasma membrane is not a hard structure, but rather a constantly moving layer that is dynamic and flexible. The lipid bilayer comprises two layers of phospholipids organised with their hydrophilic (water-loving) heads facing outward and their hydrophobic (water-fearing) tails facing inward. Its configuration permits the construction of a stable barrier that divides the interior of the cell from the exterior environment.
- The “mosaic” part of the model alludes to the fact that the plasma membrane is formed of several molecules that are embedded within the lipid bilayer. They include integral membrane proteins , which span the entire membrane width, as well as peripheral membrane proteins and lipids that are only partially entrenched in the membrane. The arrangement of the molecules within the membrane is not random, giving it a mosaic-like look.
- The Fluid Mosaic Model explains how the plasma membrane regulates the flow of materials into and out of the cell, as well as how it communicates with neighbouring cells and responds to environmental changes. Also, the concept is essential for comprehending how medications and other compounds might interact with the plasma membrane and alter cellular activity.
The plasma membrane is a dynamic and complicated structure that works as a barrier between the cell and its surroundings. It consists of multiple distinct elements, including:
- Phospholipids: These are the fundamental structural components of the plasma membrane. They create a barrier between the inside of the cell and the outside environment by forming a lipid bilayer with the hydrophobic tails facing inward and the hydrophilic heads facing outward.
- Proteins : Proteins are buried within the lipid bilayer and provide a range of activities, including as transporting substances across the membrane, recognising other cells, and facilitating communication between cells.
- Cholesterol : Cholesterol is a lipid that is present in the plasma membrane. It aids in membrane stabilisation and prevents it from becoming overly fluid or stiff.
- Glycolipids : Glycolipids are lipids that include an attached carbohydrate group. They are positioned on the membrane’s outer surface and play a function in cell recognition.
- Glycoproteins : Glycoproteins are proteins that include an attached carbohydrate group. In addition to being placed on the outside surface of the membrane, they are involved in cell recognition and communication.
- Carbohydrates : Carbohydrates are often found linked to proteins or lipids on the plasma membrane’s outer surface. They contribute to the recognition and communication of cells.
Collectively, these components comprise the plasma membrane and enable it to fulfil its many vital duties, such as regulating the exchange of materials between the cell and its environment, communicating with other cells, and preserving the structural integrity of the cell.
- According to the fluid mosaic model, the cell membrane has a quasi-fluid structure in which lipids and proteins are mosaic-arranged.
- There are two types of globular proteins: extrinsic and intrinsic. The membrane-associated extrinsic protein is soluble and dissociates. The intrinsic protein is insoluble, partially entrenched on either the outer or inner surface of the bilayer, and participates in lipid bilayer lateral diffusion .
- The fluidity of the membrane’s lipid matrix allows membrane components to migrate laterally. This is the result of the hydrophobic interactions between lipids and proteins. Fluidity is essential for a variety of membrane functions. Phospholipids and numerous intrinsic proteins contain both hydrophilic and hydrophobic groups, making them amphipathic.
- Phospholipids are complex lipids composed of glycerol, two fatty acids, and a phosphate group bound to one of numerous chemical groups. They consist of both polar (hydrophilic) and non-polar (hydrophobic) areas. The polar portion is composed of a phosphate group and glycerol, whereas the nonpolar portion is made up of fatty acids.
- All nonpolar components of the phospholipid only make contact with the nonpolar components of nearby molecules. The polar region is located outside. This trait creates the impression of a bilayer. Yet, appropriate spacing is maintained between the fatty acid chains by interspersing unsaturated chains across the membrane. This configuration preserves the semi-fluidity of the plasma membrane.
- Each phospholipid molecule’s head is water-attractive, whereas its tail is water-repellent. The hydrophilic heads of both layers of the plasma membrane face the exterior, while the hydrophobic tails constitute the interior of the bilayer. Extracellular fluid is a watery solution in which cells reside, and cells themselves contain a watery solution ( cytoplasm ). The plasma membrane forms a ring around each cell to allow the water-loving heads to be in contact with the fluid while protecting the water-averse tails on the inside.
- Principal components of a plasma membrane include lipids (phospholipids and cholesterol), carbohydrates linked to certain lipids, and proteins. A phospholipid is a molecule composed of glycerol, two fatty acids, and a phosphate-linked head group. Cholesterol, a lipid composed of four fused carbon rings, is found alongside phospholipids in the membrane’s interior.
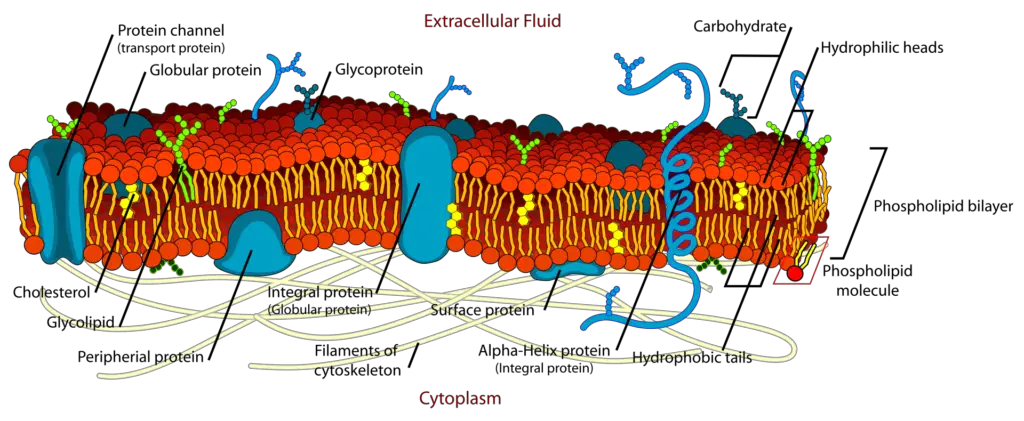
- This model was built over a long period of time by scientists from around the world. It began with the concept that the membrane was composed of a lipid bilayer, in which membrane phospholipid self-assembled into a dual layer with the hydrophobic, non-polar tails facing each other. The hydrophilic “head” sections are oriented towards the cytosol and extracellular area. This was confirmed by isolating cell membrane lipids and spreading them out in a single layer. This monolayer has double the surface area of the plasma membrane, confirming the notion that the lipids formed a bilayer.
- Yet, this was only the beginning, since it soon became clear that cell membranes required additional components to account for their diverse biophysical features. Unlike pure lipids, cell membranes are not as susceptible to freezing. The membrane’s permeability to large polar molecules could not be explained either.
- In the 1950s, more than 25 years after the lipid bilayer concept was suggested, cell membranes were first observed. Initial observations suggested that the lipid membrane was covered on both sides with thin protein sheets. Two scientists, Singer and Nicolson, developed this in 1972 to create the fluid mosaic model. In this instance, the phospholipid bilayer was claimed to be punctuated by several proteins that created a mosaic-like pattern in the lipid membrane. These proteins are capable of traversing the whole membrane or interacting with one of the two lipid layers. Some proteins were even able to bind to the membrane via a short lipid chain.
- The membrane is fluid, but has a cytoskeleton-anchored structure beneath it. The fluid nature of the lipid matrix constituting the membrane was originally demonstrated by artificially fusing membranes of different compositions. In less than an hour, the proteins of both cells redistributed themselves across the entire joined membrane.
- Certain membranes have been studied in great detail, with a resolution of less than a nanometer, using modern imaging techniques. These pictures can even disclose the relative positions of polypeptide chains and lipids within the membrane.
- Temperature : Temperature plays an important influence in determining the fluidity of the plasma membrane. The membrane becomes more fluid as the temperature rises, which can impact the stability and function of membrane proteins.
- Lipid composition: The makeup of the lipids that comprise the plasma membrane can also have an effect on its fluidity. The fluidity of lipids with unsaturated fatty acid tails is typically greater than that of lipids with saturated fatty acid tails.
- Cholesterol content: Cholesterol content is an additional element that affects the fluidity of the plasma membrane. At low temperatures, cholesterol works as a buffer and keeps the membrane from becoming excessively rigid, but at high temperatures, it stabilises the membrane and prevents it from becoming excessively fluid.
- Membrane protein content: The presence of membrane proteins can also influence the fluidity of the plasma membrane. The size and form of proteins can inhibit the fluidity of the surrounding lipids, resulting in a less fluid membrane.
- Cytoplasmic and extracellular environment: The chemical and physical features of the intracellular and extracellular environments can also have an effect on the fluidity of the plasma membrane. For instance, excessive salt concentrations might reduce membrane mobility, but a low pH can enhance it.
- They cause compartmentalization by separating the cells from their external environment. As organelle coverings, they enable the organelles to maintain their identity, internal environment, and functional diversity.
- The plasma membrane safeguards the cell from harm.
- The cell membrane permits the exchange of materials and information between organelles within the same cell as well as between cells.
- The selective permeability of cell membranes allows only certain chemicals to enter inward to varying degrees. Others are impenetrable to the membranes.
- On the surface of the plasma membrane are particular chemicals that serve as recognition centres and attachment sites. This allows WBCs to distinguish between germ and body cells.
- It offers a permeability barrier, preventing the exit of cellular material from the cell, while allowing the selective admission of organic and inorganic molecules. Hence, plasma membranes demonstrate selective permeability.
The fluid mosaic model is a model that describes the structure of the cell membrane. It suggests that the membrane is composed of a fluid-like lipid bilayer with embedded proteins that can move laterally within the membrane.
The fluid mosaic model was proposed by S. J. Singer and Garth Nicolson in 1972.
The fluid mosaic model consists of a phospholipid bilayer, embedded proteins, and cholesterol.
Molecules move within the membrane by diffusion, which occurs because of the fluid nature of the lipid bilayer.
Cholesterol helps to maintain the fluidity of the membrane by preventing the phospholipid tails from packing too closely together.
Proteins embedded in the membrane include transmembrane proteins, which span the entire membrane, and peripheral proteins, which are attached to the surface of the membrane.
Proteins are able to move laterally within the membrane because they are not covalently bound to the lipid bilayer.
The fluid mosaic model provides an explanation for the membrane’s ability to act as a selectively permeable barrier while still allowing for the transport of molecules across the membrane.
The fluidity of the membrane is affected by temperature, lipid composition, and the presence of cholesterol.
The fluid mosaic model has been supported by various experiments, including the use of freeze-fracture electron microscopy and fluorescence recovery after photobleaching (FRAP).
- https://ib.bioninja.com.au/standard-level/topic-1-cell-biology/13-membrane-structure/fluid-mosaic-model.html
- https://www.jove.com/science-education/10698/the-fluid-mosaic-model
- https://www.biologyonline.com/dictionary/fluid-mosaic-model
- https://www.khanacademy.org/science/ap-biology/cell-structure-and-function/membrane-permeability/a/fluid-mosaic-model-cell-membranes-article
Related Biology Study Notes
Cancer cell – definition, types, morphology, development, cell differentiation – definition, process & examples, gamete – types, formation, functions, examples, egg cell – structure, types, functions, osmosis – definition, types, mechanism, significance, examples, membrane permeability – definition, factors affects, examples, checkpoints in the cell cycle – g1, g2, metaphase (spindle) checkpoints, cell cycle – definition, phases, checkpoints, regulation, latest questions.
- All Questions
Start Asking Questions Cancel reply
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
- Click on your ad blocker icon in your browser's toolbar
- Select "Pause" or "Disable" for this website
- Refresh the page if it doesn't automatically reload

Fluid Mosaic Model: Definition, Discovery, Components, Structure And Functions
Fluid mosaic model definition.
The fluid mosaic model defines the structure of cell membranes as a fluid phospholipid bilayer embedded with various proteins in a mosaic-like pattern. The membrane is fluid, allowing the components to move laterally, and contains integral proteins embedded within the membrane and peripheral proteins attached to the surface.
What Is The Fluid Mosaic Model?
The fluid mosaic model is a scientific model that describes the structure and behavior of biological membranes , particularly the plasma membrane that surrounds cells. It explains how the cell membrane is a dynamic and fluid structure composed of various molecules arranged in a mosaic-like pattern.
Discovery Of Fluid Mosaic Model
The fluid mosaic model was proposed in 1972 by two scientists, S.J. Singer and Garth L. Nicolson, based on their research and observations of the cell membrane structure.
Historical Timeline Of Fluid Mosaic Model
- 1920s : Gorter and Grendel proposed that phospholipids in the cell membrane are arranged in a bilayer .
- 1930s : Davson and Danielli suggested that proteins are arranged in layers above and below the phospholipid bilayer.
- 1972 : Singer and Nicolson proposed the fluid mosaic model , which described the cell membrane as a fluid structure with proteins embedded in a phospholipid bilayer.
- Present : The fluid mosaic model has been refined and updated based on new discoveries, but it remains the most widely accepted model for describing the structure and behavior of biological membranes.
Why Is It Called Fluid Mosaic Model?
The cell membrane structure is called the fluid mosaic model for two main reasons:
- Fluidity: The membrane is fluid, with individual lipid and protein molecules able to diffuse rapidly and freely in the plane of the membrane. The membrane lipids are constantly moving laterally, giving membranes a dynamic, fluid structure rather than a static, fixed one. This fluidity is affected by temperature, fatty acid tail saturation, and cholesterol content.
- Mosaic structure: The membrane has a mosaic-like composition of diverse components, including phospholipids, cholesterol, proteins, and carbohydrates. These components are distributed non-uniformly in the membrane, forming a pattern resembling a mosaic tile artwork. Integral proteins are embedded at various places in the fluid lipid bilayer.
Fluid Mosaic Model Diagram
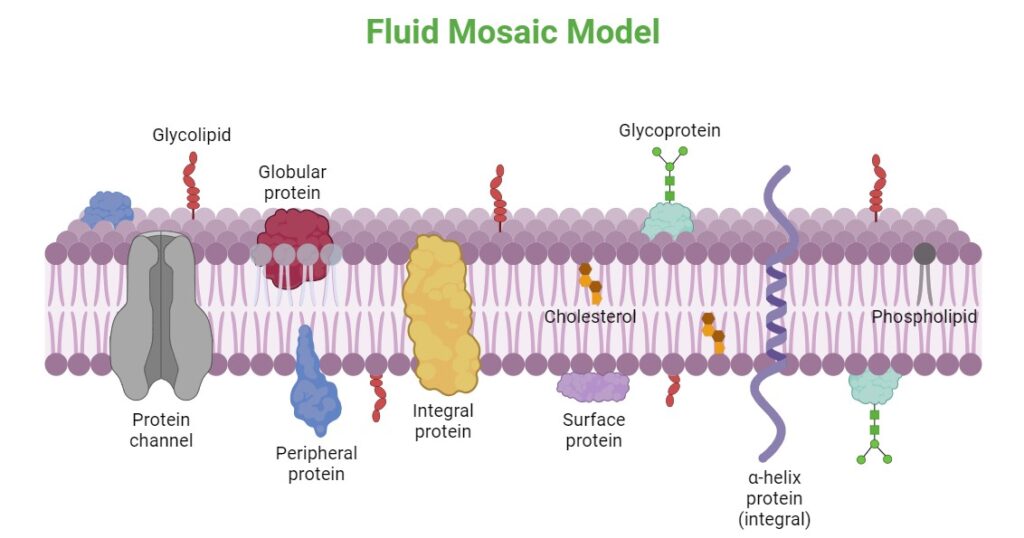
Fluid Mosaic Model Structure
The fluid mosaic model describes the cell membrane as a phospholipid bilayer with various components embedded or attached to it:
- Phospholipids : The main fabric of the membrane, forming a bilayer with their hydrophobic tails facing inward and hydrophilic heads facing outward.
- Cholesterol : Molecules found between the phospholipids, helping to maintain membrane fluidity.
- Integral proteins : Proteins that are embedded within the phospholipid bilayer, either partially or completely spanning the membrane.
- Peripheral proteins : Proteins that are loosely attached to the surface of the membrane, either on the inner or outer side.
- Carbohydrates : Attached to some proteins and lipids, forming glycoproteins and glycolipids , respectively.
Components Of Fluid Mosaic Model
The fluid mosaic model consists of the following main elements:
- Phospholipids : Amphipathic molecules with a hydrophilic head and hydrophobic tails , forming the basic structure of the membrane.
- Cholesterol : Steroid molecules that help maintain membrane fluidity and permeability.
- Carbohydrates : Attached to some proteins and lipids, forming glycoproteins and glycolipids, respectively.
What Are The Functions Of Fluid Mosaic Model?
The fluid mosaic model explains various functions of the cell membrane , including:
- Selective permeability : The membrane acts as a barrier, allowing only specific molecules to pass through while blocking others.
- Cell signaling : Proteins embedded in the membrane can act as receptors for signaling molecules, enabling communication between cells.
- Enzyme activity : Some membrane proteins have enzymatic functions, catalyzing chemical reactions.
- Cell recognition : Carbohydrates on the membrane surface can act as markers for cell recognition and adhesion.
- Transport : Membrane proteins facilitate the transport of molecules across the membrane through various mechanisms, such as diffusion, facilitated diffusion, and active transport.
Why Fluid Mosaic Model Important For Cell Membrane?
The fluid mosaic model is important for the cell membrane because:
- The fluid mosaic model explains the dynamic and fluid nature of the membrane, allowing for lateral movement of components.
- The mosaic structure of different molecules , including phospholipids, proteins, and carbohydrates.
- Selective permeability of the membrane, allows some molecules to pass through while blocking others.
- Fluid mosaic structure helps cell signaling, transport, and enzyme activity in cell membranes.
What Part Of The Cell Membrane Acts Like A Fluid?
In the fluid mosaic model, the phospholipid bilayer acts like a fluid. The phospholipid molecules are not rigidly fixed in place but can move laterally within the membrane, giving it a fluid-like property.
What Is Meant By Membrane Fluidity?
Membrane fluidity refers to the ability of the components within the cell membrane to move and change positions. It describes the dynamic nature of the membrane, where molecules can move laterally and rotate within the plane of the membrane.
Why Is Membrane Fluidity Important?
Membrane fluidity is important for several reasons:
- Selective permeability : The fluidity of the membrane allows for the movement of specific molecules across the membrane through protein channels or carriers.
- Cell signaling : Membrane fluidity enables the movement and clustering of receptors and signaling molecules, facilitating cell communication.
- Enzyme activity : Many enzymes are embedded in the membrane, and their activity depends on the fluidity of the membrane environment.
- Cell fusion and division : Membrane fluidity is essential for processes like cell fusion, cell division, and vesicle formation, where the membrane needs to be flexible and dynamic.
- Adaptation to temperature changes : Organisms can adjust the fluidity of their membranes by altering the lipid composition to adapt to different temperature conditions.
Why Is The Fluid Mosaic Model Most Accepted?
There are number of reasons why is the fluid mosaic model is the most widely accepted model for cell membranes:
- Experimental evidence : Various experimental techniques, such as electron microscopy, X-ray diffraction, and fluorescence microscopy, have provided evidence supporting the fluid mosaic model.
- Explanatory power : The model can explain various membrane-related phenomena, such as selective permeability, cell signaling, and transport processes.
- Adaptability : The model has been refined and updated over time to incorporate new discoveries and observations, making it flexible and adaptable.
- Simplicity : Despite its complexity, the fluid mosaic model provides a simple and intuitive representation of the membrane structure, making it easier to understand and communicate.
What Advantage Does A Cell Membrane Have Being A Fluid Mosaic Model?
The fluid mosaic model of the cell membrane provides numerous advantages:
- The lateral movement of membrane components allows for dynamic processes, such as cell signaling, transport, and membrane fusion/fission.
- The mosaic arrangement of proteins and lipids enables the selective passage of molecules across the membrane.
- The membrane can adapt to changes in temperature and environmental conditions by altering its lipid composition and protein distribution.
- The membrane separates the cell from its environment and maintains internal compartmentalization , enabling different biochemical processes to occur simultaneously.
- The presence of specific proteins and carbohydrates on the membrane surface allows for cell recognition and adhesion processes.
What Are The Disadvantages Of The Fluid Mosaic Model?
While the fluid mosaic model is widely accepted and useful, it does have some limitations and disadvantages:
- Oversimplification : The model simplifies the complex structure and dynamics of biological membranes, which can be more intricate in reality.
- Lack of detail : The model does not provide detailed information about the specific interactions and arrangements of membrane components.
- Static representation : The model represents a snapshot of the membrane structure, while in reality, the membrane is highly dynamic and constantly changing.
- Membrane asymmetry : The model does not fully account for the asymmetric distribution of lipids and proteins between the inner and outer leaflets of the membrane.
- Membrane domains : The model does not explicitly address the existence of specialized membrane domains, such as lipid rafts, which have been discovered through more recent research.
Read: Plant Cell – Definition, Structure, Types, Functions, and Important
FAQs Of Fluid Mosaic Model
Who proposed the fluid mosaic model.
The fluid mosaic model was proposed in 1972 by S.J. Singer and Garth L. Nicolson, two scientists who studied the structure and behavior of biological membranes.
What Role Do Phospholipids Play In The Fluid Mosaic Model?
Phospholipids form the basic structure of the membrane, creating a bilayer with hydrophilic heads facing outward and hydrophobic tails facing inward, providing a barrier that is selectively permeable.
How Does Cholesterol Affect The Fluidity Of The Membrane
Cholesterol is interspersed among the phospholipids and helps to maintain membrane fluidity by preventing the fatty acid chains from packing too closely in cold temperatures and by restraining excessive movement in warm temperatures.
How Do Carbohydrates Contribute To The Fluid Mosaic Model?
Carbohydrates are attached to proteins (forming glycoproteins) or lipids (forming glycolipids) on the extracellular surface of the membrane. They play a crucial role in cell recognition, signaling, and adhesion.
Similar Posts
Why Cell Wall Is Important For Plant Cell?
The cell wall is crucial for plant cells as it provides structural support and protection. It allows the plant cell to withstand internal turgor pressure and maintain its characteristic rectangular shape. The cell wall also regulates growth by selectively loosening during cell expansion and division. There are several reasons why the cell wall is important…
Top 10 Plant Cell Organelles: Structure Functions, and Components
Hello, plant sciences lovers. Welcome to this article on plant cell organelles. In this article, I will cover: Let’s dive deep. What Are Plant Cell Organelles? Plant cell organelles are specialized structures within plant cells that perform specific functions. These tiny components work together to keep the cell alive and functioning properly. Each organelle has…

Cell Wall: Definition, Structure, Compositions, And Functions
Cell Wall Definition A cell wall is a protective layer that surrounds the cells of plants, fungi, bacteria, and some other organisms. It is located just outside the cell membrane. The cell wall provides the cell with strength, rigidity, and protection against mechanical stress and cell wall helps maintain the shape of the cell as…
How Ion Transport Through Plant Cell Membranes
Ion transport through plant cell membranes is a crucial process that enables plants to maintain proper cellular function, respond to environmental stimuli, and regulate growth and development. Ions, such as potassium (K+), sodium (Na+), calcium (Ca2+), and chloride (Cl–), play essential roles in various aspects of plant physiology, including osmotic regulation, signal transduction, and enzyme…
How Cell Wall Is Formed In Plant Cells?
During cell division, a structure called the cell plate is formed from Golgi-derived vesicles containing cell wall materials like cellulose, hemicellulose, and pectin. The cell plate expands outward and eventually fuses with the existing cell wall, separating the two daughter cells and forming the new primary cell wall between them. As the cell matures, some…
Cell Membrane (Plasma Membrane): Definition, Structure, Composition and Functions
What Is Cell Membrane? The cell membrane, also known as the plasma membrane, is a thin, semi-permeable barrier that surrounds and encloses the contents of a cell. It separates the interior of the cell from the external environment, protecting the cell and regulating the movement of substances in and out of the cell. Cell Membrane…

Microbe Notes
Fluid Mosaic Model
- The plasma membrane, also known as the cell surface membrane or plasmalemma, defines the boundary of the cell.
- They are a special type of membranes which are lipid structures that separate the cell from its environment.
- In composition, it is a phospholipid bilayer with embedded proteins that enclosing every living cell.
- It serves some specific functions such as controlling the flow of nutrients and ions into and out of the cells, mediating the response of a cell to external stimuli (a process called signal transduction), and interacting with bordering cells.
Table of Contents
Interesting Science Videos
The Fluid Mosaic Model of Biomembranes
- The fluid mosaic model describes the structure of the plasma membrane as a mosaic of components —including phospholipids, cholesterol, proteins, and carbohydrates—that gives the membrane a fluid character.
- Membranes are impermeable to most polar or charged solutes, but permeable to nonpolar compounds; they are 5 to 8 nm (50 to 80 Å) thick and appear trilaminar when viewed in cross-section with the electron microscope.
- The combined evidence from electron microscopy and studies of chemical composition, as well as physical studies of permeability and the motion of individual protein and lipid molecules within membranes, led to the development of the fluid mosaic model for the structure of biological membranes.
- A bilayer of phospholipids about 3 nm thick provides the basic architecture of all cellular membranes with membrane proteins giving each cellular membrane its unique set of functions.
- Thus the plasma membrane consists of a lipid bilayer containing embedded and peripheral proteins. The major component of membranes is, however, lipids.
- Individual phospholipids can move laterally and spin within the plane of the membrane, giving the membrane a fluid-like consistency similar to that of olive oil.
- Membrane lipids are strongly amphipathic molecules with a polar hydrophilic “head group” and a polar hydrophobic “tail.”
- The polar head group attached to two hydrophobic fatty acid tails; the head group faces the aqueous environment, the fatty acid tails the interior of the bilayer.
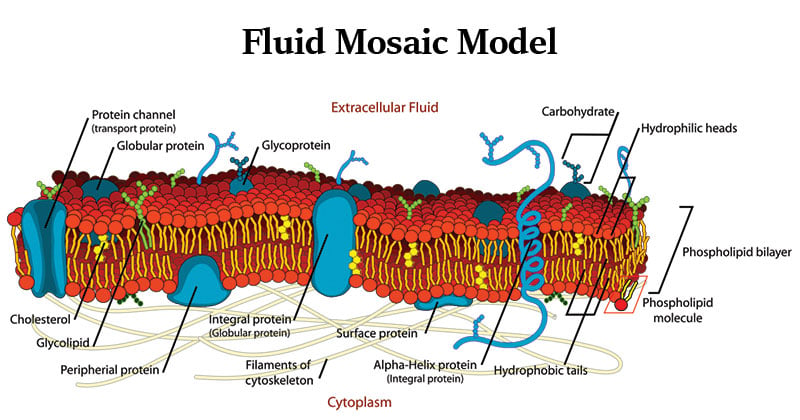
Figure: Fluid mosaic model of a cell membrane, Source: Wikipedia.
- In membranes, they are primarily held together by the hydrophobic effect and weak Van der Waals forces and are therefore mobile relative to each other. This gives membranes a more or less fluid quality.
- Non- covalent interactions between phospholipids, and between phospholipids and proteins, also lend strength and resilience to the membrane.
- At the same time, the hydrophobic core of the bilayer prevents the unassisted movement of water-soluble substances from one side to the other.
- Integral membrane proteins (transmembrane proteins) span the bilayer and often form dimers and higher-order oligomers.
- Lipid-anchored proteins are tethered to one leaflet by a covalently attached hydrocarbon chain.
- Peripheral proteins associate with the membrane primarily by specific non- covalent interactions with integral membrane proteins or membrane lipids.
- Proteins in the plasma membrane also make extensive contact with the cytoskeleton.
- Lipids and proteins are mobile within the membrane. If they are not fixed in place by special mechanisms, they float within the lipid layer as if in a two-dimensional liquid; biological membranes are therefore also described as being a “fluid mosaic”.
- The carbohydrate moieties attached to some proteins and lipids of the plasma membrane are exposed on the extracellular surface of the membrane.
- Lodish, H. F., Berk, A., Kaiser, C., Krieger, M., Scott, M. P., Bretscher, A., Ploegh, H. L., Matsudaira, P. T. (2008). Molecular cell biology. New York: W.H. Freeman.
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- Koolman, J., & Röhm, K.-H. (2005). Color atlas of biochemistry. Stuttgart: Thieme.
- Alberts, B. (2004). Essential cell biology. New York, NY: Garland Science Pub.
- https://www.mheducation.co.uk/he/chapters/9780071102087.pdf
- http://www.nslc.wustl.edu/courses/bio101/cruz/Organelles/Organelle.htm
About Author
Sagar Aryal
1 thought on “Fluid Mosaic Model”
How can i seperate its properties from its functions and structure
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .

IMAGES
COMMENTS
Fluid mosaic model of a cell membrane. The fluid mosaic model explains various characteristics regarding the structure of functional cell membranes.According to this biological model, there is a lipid bilayer (two molecules thick layer consisting primarily of amphipathic phospholipids) in which protein molecules are embedded.
Apr 27, 2017 · The fluid mosaic model was refined in the early 1980s, by two scientists called Mouritsen and Bloom to create the ‘mattress model’ for membrane structure. They demonstrated the fact that while earlier experiments had suggested that the entire membrane is fluid and allows free diffusion of proteins, there are in fact, subdomains within each ...
Nov 23, 2024 · The fluid mosaic model was first proposed by S.J. Singer and Garth L. Nicolson in 1972 to explain the structure of the plasma membrane. The model has evolved somewhat over time, but it still best accounts for the structure and functions of the plasma membrane as we now understand them.
The essential elements of the Fluid–Mosaic Membrane Model have proven to be remarkably consistent with experimental results on the fundamental properties of biological membranes, but it was inevitable that the original model could not explain all of the properties of membrane structure and dynamics found in various cellular membranes [18,19 ...
Jul 21, 2023 · Fluid Mosaic Model Definition. What is the fluid mosaic model? The fluid mosaic model is a three-dimensional representation of the structure and dynamics of the plasma membrane proposed by S.J. Singer and G.L. Nicolson in 1972. The fluid mosaic model describes the plasma membrane as a ‘fluid` and a ‘mosaic’ structure.
Feb 1, 2023 · The fluid mosaic model is a way biologists use to describe the structure of biological membranes, such as the cell membrane. It was first proposed by Seymour Jonathan Singer and Garth L. Nicolson in 1972. The model has been modified in parts over time, keeping the basic concept the same.
Apr 7, 2024 · The Fluid Mosaic Model of Plasma Membrane. According to the fluid mosaic model, the cell membrane has a quasi-fluid structure in which lipids and proteins are mosaic-arranged. There are two types of globular proteins: extrinsic and intrinsic. The membrane-associated extrinsic protein is soluble and dissociates.
May 25, 2024 · Discovery Of Fluid Mosaic Model. The fluid mosaic model was proposed in 1972 by two scientists, S.J. Singer and Garth L. Nicolson, based on their research and observations of the cell membrane structure. Historical Timeline Of Fluid Mosaic Model. 1920s: Gorter and Grendel proposed that phospholipids in the cell membrane are arranged in a bilayer.
Apr 5, 2022 · Figure: Fluid mosaic model of a cell membrane, Source: Wikipedia. In membranes, they are primarily held together by the hydrophobic effect and weak Van der Waals forces and are therefore mobile relative to each other. This gives membranes a more or less fluid quality.
May 26, 2021 · The fluid mosaic model was first proposed by S.J. Singer and Garth L. Nicolson in 1972 to explain the structure of the plasma membrane. The model has evolved somewhat over time, but it still best accounts for the structure and functions of the plasma membrane as we now understand them.