
Fraenkel Conrat Experiment
Fraenkel Conrat experiment was based on the evidence that RNA also carry genetic information. This was the very first experiment that was introduced to prove that RNA is also a genetic material with the help of TMV. Tobacco Mosaic Virus is composed of 6% of RNA (not DNA) and protein.
Frankel-Conrat and co-workers determined that the reconstitution of infective virus particles may occur after the association of protein subunits and RNA. Before this experiment, there were many experimental proofs that proved DNA being a genetic material. So, it was difficult for Fraenkel to prove RNA also carries a hereditary material.
Content: Fraenkel Conrat Experiment
Experimental organism.
In the year 1957, Fraenkel Conrat and Singer reconstituted viruses after mixing mutant strain’s protein with the other strain’s RNA. As a result, the new virus particles produced by the infected host plant that has the protein type produced by the RNA parent.
Fraenkel Conrat was famous for his viral research, where he studied that in some viruses (like TMV or HRV), RNA is the controlling factor for viral reproduction or reconstitution.
Fraenkel-Conrat (1957) conducted experiments on TMV to demonstrate that a few viruses contain RNA. TMV or Tobacco Mosaic Virus is a small plant virus that causes infection in solanaceous plants that appear as in the mosaic pattern.
Tobacco mosaic virus comprises a single coiled RNA encapsulated in a cylindrical protein coat. Different strains of TMV are characterized by differences in the chemical composition of their protein coats.
Step-1 : Fraenkel Conrat first developed techniques to isolate proteins and RNA of TMV by using the appropriate chemical treatments. After isolation, it was observed that the protein alone did not cause infection in the tobacco leaves. In contrast, the separated RNA molecule was sufficient enough to cause mosaic in the tobacco leaves.
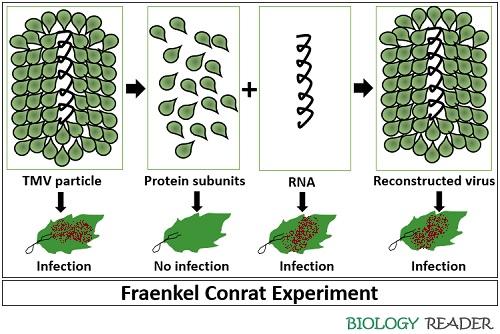
Step-2 : Then Fraenkel Conrat reversed the process by mixing the protein and RNA under appropriate conditions. After mixing the protein subunits or capsomers of TMV with the RNA molecule, he observed the reconstitution or formation of complete infective TMV particles.
Step-3 : In the third experiment, Fraenkel-Conrat and Singer took two different strains of TMV (type-A and type-B). Then they separated the RNAs from the protein coats. After that, both Fraenkel-Conrat and Singer mixed proteins of one strain with the RNA of the second strain to reconstitute hybrid viruses.
After rubbing the hybrid or reconstituted viruses onto live tobacco leaves, the phenotypically and genotypically identical progeny viruses were produced similar to the parental type from which the RNA had been isolated.
Therefore, by all these experiments, Fraenkel Conrat concluded that both DNA and RNA carries genetic information. By his experiments, it was proved that the genetic information of TMV is stored in the RNA and not in the protein.
However, DNA perhaps always function as genetic material, but RNA, in most cases, is non-genetic. Only in specific systems, where the DNA is absent, RNA function as hereditary material.
Related Topics:
- Citrus Canker Disease
- Microbial Food Spoilage
- Production of Citric Acid
- Oxidase Test
- Activated Sludge Treatment
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Start typing and press enter to search
A Smithsonian magazine special report
How a Few Sick Tobacco Plants Led Scientists to Unravel the Truth About Viruses
With the COVID-19 coronavirus causing a global pandemic, a look back at the scientists who figured out viruses and their relationship to disease
Theresa Machemer
Correspondent
:focal(548x458:549x459)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/27/3c/273ca1f6-8f61-4f1a-bdcf-46f274ebb87d/tobaccomosaicvirus.jpg)
When German pathologist Robert Koch discovered the bacterium behind tuberculosis in 1882, he included a short guide for linking microorganisms to the diseases they cause. It was a windfall for germ theory, the modern understanding that pathogens can make us sick. But it didn’t only shake up the field of medicine: Botanists took note, too.
When a blight of mosaic disease threatened European tobacco crops in the mid-1800s, plant pathologists set out to identify its root cause. For decades, only one forward-thinking botanist, Martinus Beijerinck, realized the source was neither a bacterial nor a fungal infection, but something completely different: a virus.
Today, we know that viruses can be found nearly anywhere in the air , oceans and soil . A tiny percentage of these are dangerous pathogens that cause disease, such as the current coronavirus called SARS-CoV-2 causing a worldwide pandemic. Yet the study of viruses started not in medical science, but in botany, the study of plants. Viruses are so small—and so strange—that it would take decades for scientific consensus to agree that they exist at all.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/f4/1a/f41af8b0-6e90-4e28-9125-ec87d7512123/former_delft_school_of_microbiology.jpg)
Agents of Disease
The idea that microorganisms could cause plant disease wasn’t entirely new even in the late 19th century. In the 1840s, Reverend Miles Berkeley, also a botanist, identified the fungus behind Ireland’s potato blight, despite the clergy’s notion that the devil was to blame.
In 1857, farmers in the Netherlands reported a disease threatening another economically vital crop: tobacco. The leaves began turning a mottled dark green, yellow, and grey, causing farmers to lose up to 80 percent of crops in affected fields. Massive fields of tobacco that had been planted with the same crop repeatedly were especially susceptible . Once the disease reached a farmer’s field, it spread rapidly.
“It's very easy for it to move around,” says plant virologist Karen-Beth Scholthof of Texas A&M University. “If you're in a greenhouse or your garden and you're watering with a hose and the hose touches an affected plant, you can end up damaging a plant next to it.”
In the Netherlands, plant pathologist Adolf Mayer began researching the disease in 1879 and named it the “mosaic disease of tobacco.” He tried to use Koch’s guidelines, which call for a series of germ isolations and re-infections, to find its cause. But Mayer ran into trouble. Although he showed that the sap from a sick tobacco leaf could pass the disease to a healthy leaf, he couldn’t produce a pure culture of the pathogen and couldn’t spot the culprit under a microscope.
“The tools did not exist to see a virus,” says biological anthropologist Sabrina Sholts , curator of the Smithsonian National Museum of Natural History’s Outbreak exhibit. “It was just this invisible contagion.”
When botanist Dmitri Ivanovski researched tobacco mosaic disease in Crimea beginning in 1887 , he took a different approach. He strained the sap through fine filters made of unglazed porcelain, a material with pores that were too small for bacteria to squeeze through. But when Ivanovski put the filtered sap on a healthy tobacco leaf, it turned mottled yellow with disease. Ivanovski could barely believe his data, which he published in 1892. He concluded that the disease was caused by a toxin that fit through the filter or that some bacteria had slipped through a crack.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/7e/fa/7efaa029-a70f-4761-85e9-6d298a07060d/screen_shot_2020-03-20_at_10422_pm.png)
Dutch microbiologist Beijerinck independently conducted almost the same experiments as Ivanovski, but he came to a much different conclusion. The early pathologist added to the porcelain filter experiments with a second kind of filtration system that used a gelatin called agar to prove that no microorganisms survived the first filtration. Bacteria get stuck on top of the gelatin, but the mysterious mosaic-causing pathogen diffused through it.
Beijerinck also provided evidence that the disease agent relies on growing leaves to multiply. By re-filtering the pathogen from an infected leaf and using it to cause mosaic disease on another plant, he showed that the agent could spread without diluting its disease-causing power. He proved the pathogen was growing in the leaves, but strangely, it couldn’t reproduce without them.
When he published his findings in 1898, Beijerinck called the infectious, filtered substance contagium vivum fluidum— a contagious, living fluid. As a shorthand, he reintroduced the word “virus” from the Latin for a liquid poison to refer specifically to this new kind of pathogen.
“I don't think Ivanovski really understood his results,” Scholthof says. “Beijerinck set up the experiments and trusted what he saw… The way we use ‘virus’ today, he was the first one to bring that term to us in a modern context, and I would give him credit for the beginning of virology.”
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/fd/3e/fd3edbef-a377-4ad0-b17a-375cec4b5935/screen_shot_2020-03-20_at_10410_pm.png)
A Bold Hypothesis
Although Beijerinck incorrectly thought viruses were liquid (they are particles) his results were close to the mark. Yet his idea didn’t catch on. His suggestion of a pathogen without a cell conflicted with early germ theory and was radical for the time.
Ivanovski continued to search for a bacterial cause of tobacco mosaic disease, claiming “that the entire problem will be solved without such a bold hypothesis ” as Beijerinck’s. In the meantime, researchers grappled with the evidence at hand. In 1898, the same year as Beijerinck’s work was published, foot-and-mouth disease in cattle became the first animal illness linked to a filterable agent, or a microbe small enough to pass through a porcelain filter. In 1901, American researchers studying yellow fever in Cuba concluded that the disease carried by mosquitoes was caused by something small enough to be filterable , too.
At the time, the researchers didn’t consider their discoveries to be viruses like Beijerinck’s. The prevailing theory was that there were simply bacterial that could fit through the filter. Early review articles of invisible contagions sometimes grouped barely visible bacteria with Beijerinck’s viruses.
“In the early days, there was a lot of confusion because you couldn’t see them,” Scholthof says. Questions about whether these tiny germs were small bacteria, molecules secreted by bacteria, or something else remained unanswered into the 1920s. “Some people would probably say [the questions went on] until they could be seen with an electron microscope,” she says.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/96/64/96640672-80db-41ee-ac46-618e73a3ad19/covid19.jpg)
A Model Virus
In 1929, biologist Francis Holmes used the tobacco mosaic virus to develop a method proving that viruses are discrete particles mixed in the filtered sap and that they have stronger effects at higher concentrations. In 1935, chemist Wendell M. Stanley created a crystallized sample of the virus that could be visualized with X-rays, earning him a share of the 1946 Nobel Prize. (The clearest X-ray diffraction image of tobacco mosaic virus came from Rosalind Franklin, in 1955 , after her contributions to the discovery of DNA’s double helix.) The first clear, direct photographs of tobacco mosaic virus would not come until 1941 with the invention of powerful electron transmission microscopes, which revealed the pathogen’s skinny, sticklike shape.
This was a turning point in the scientific understanding of viruses because visual proof dispelled any doubt of their existence. The images showed that viruses are simple structures made of genetic material wrapped in a solid coat of protein molecules—a far cry from squishy, cellular bacteria. But Beijerinck didn’t live to see his theory validated, as he died in 1931.
“In a way, we were lucky that it was this was a disease found on tobacco,” Scholthof says. “It was an economic problem. It was easy to work with and purify. The virus itself only in it encodes five genes.” Because the virus has been a research subject for so long, it was used to develop fundamental ideas in virology. It remains a tool in plant virology today.
Mayer, Ivanovski and Beijerinck’s work didn’t stop the spread of tobacco mosaic during their lifetime; tobacco production halted entirely in the Netherlands. But their pioneering work on tobacco mosaic virus opened the door to a century of research that has revealed a diverse range of viral structures and strategies for survival.
While tobacco mosaic virus is rod-shaped and made up only of genes and protein, others, like the COVID-19 coronavirus, are round and wrapped in a fatty envelope that makes them especially susceptible to soap when you wash your hands . Advancements in the understanding of how viruses spread allowed for the eradication of smallpox and the invention of several life-saving vaccinations.
“It's only been in the last century that a lot of these amazing achievements happened, and it's happened so fast and so dramatically that we almost can't relate to what the world was like,” Sholts says. Right now, “there's a lot to be concerned about and take seriously. But I usually find what the scientists are doing to be one of the brightest elements to anything that you might look at.”
Get the latest Science stories in your inbox.
Theresa Machemer | READ MORE
Theresa Machemer is a freelance writer based in Washington DC. Her work has also appeared in National Geographic and SciShow. Website: tkmach.com
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Milestones in the research on tobacco mosaic virus.
B d harrison.
- Author information
- Copyright and License information
Beijerinck's (1898) recognition that the cause of tobacco mosaic disease was a novel kind of pathogen became the breakthrough which eventually led to the establishment of virology as a science. Research on this agent, tobacco mosaic virus (TMV), has continued to be at the forefront of virology for the past century. After an initial phase, in which numerous biological properties of TMV were discovered, its particles were the first shown to consist of RNA and protein, and X-ray diffraction analysis of their structure was the first of a helical nucleoprotein. In the molecular biological phase of research, TMV RNA was the first plant virus genome to be sequenced completely, its genes were found to be expressed by cotranslational particle disassembly and the use of subgenomic mRNA, and the mechanism of assembly of progeny particles from their separate parts was discovered. Molecular genetical and cell biological techniques were then used to clarify the roles and modes of action of the TMV non-structural proteins: the 126 kDa and 183 kDa replicase components and the 30 kDa cell-to-cell movement protein. Three different TMV genes were found to act as avirulence genes, eliciting hypersensitive responses controlled by specific, but different, plant genes. One of these (the N gene) was the first plant gene controlling virus resistance to be isolated and sequenced. In the biotechnological sphere, TMV has found several applications: as the first source of transgene sequences conferring virus resistance, in vaccines consisting of TMV particles genetically engineered to carry foreign epitopes, and in systems for expressing foreign genes. TMV owes much of its popularity as a research mode to the great stability and high yield of its particles. Although modern methods have much decreased the need for such properties, and TMV may have a less dominant role in the future, it continues to occupy a prominent position in both fundamental and applied research.
The Full Text of this article is available as a PDF (193.3 KB).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERER F. A. Preparation and properties of an artificial antigen immunologically related to tobacco mosaic virus. Biochim Biophys Acta. 1963 Apr 2;71:246–248. doi: 10.1016/0006-3002(63)91077-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- ANDERER F. A., UHLIG H., WEBER E., SCHRAMM G. Primary structure of the protein of tobacco mosaic virus. Nature. 1960 Jun 18;186:922–925. doi: 10.1038/186922a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Abel P. P., Nelson R. S., De B., Hoffmann N., Rogers S. G., Fraley R. T., Beachy R. N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science. 1986 May 9;232(4751):738–743. doi: 10.1126/science.3457472. [ DOI ] [ PubMed ] [ Google Scholar ]
- Butler P. J., Finch J. T., Zimmern D. Configuration of tobacco mosaic virus, RNA during virus assembly. Nature. 1977 Jan 20;265(5591):217–219. doi: 10.1038/265217a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Butler P. J., Klug A. Assembly of the particle of tobacco mosaic virus from RNA and disks of protein. Nat New Biol. 1971 Jan 13;229(2):47–50. doi: 10.1038/newbio229047a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Citovsky V., Knorr D., Schuster G., Zambryski P. The P30 movement protein of tobacco mosaic virus is a single-strand nucleic acid binding protein. Cell. 1990 Feb 23;60(4):637–647. doi: 10.1016/0092-8674(90)90667-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- Dawson W. O., Beck D. L., Knorr D. A., Grantham G. L. cDNA cloning of the complete genome of tobacco mosaic virus and production of infectious transcripts. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1832–1836. doi: 10.1073/pnas.83.6.1832. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fraenkel-Conrat H., Williams R. C. RECONSTITUTION OF ACTIVE TOBACCO MOSAIC VIRUS FROM ITS INACTIVE PROTEIN AND NUCLEIC ACID COMPONENTS. Proc Natl Acad Sci U S A. 1955 Oct 15;41(10):690–698. doi: 10.1073/pnas.41.10.690. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- GIERER A., MUNDRY K. W. Production of mutants of tobacco mosaic virus by chemical alteration of its ribonucleic acid in vitro. Nature. 1958 Nov 22;182(4647):1457–1458. doi: 10.1038/1821457a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- GIERER A., SCHRAMM G. Infectivity of ribonucleic acid from tobacco mosaic virus. Nature. 1956 Apr 14;177(4511):702–703. doi: 10.1038/177702a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gallie D. R., Sleat D. E., Watts J. W., Turner P. C., Wilson T. M. The 5'-leader sequence of tobacco mosaic virus RNA enhances the expression of foreign gene transcripts in vitro and in vivo. Nucleic Acids Res. 1987 Apr 24;15(8):3257–3273. doi: 10.1093/nar/15.8.3257. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gianinazzi S., Martin C., Vallée J. C. Hypersensibilité aux virus, température et protéines soubles chez le Nicotiana Xanthi n.c. Apparition de nouvelles macromolécules lors de la répression de la synthèse virale. C R Acad Sci Hebd Seances Acad Sci D. 1970 May 11;270(19):2383–2386. [ PubMed ] [ Google Scholar ]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- HARRIS J. I., KNIGHT C. A. Action of carboxypeptidase on tobacco mosaic virus. Nature. 1952 Oct 11;170(4328):613–614. doi: 10.1038/170613a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Harrison B. D., Finch J. T., Gibbs A. J., Hollings M., Shepherd R. J., Valenta V., Wetter C. Sixteen groups of plant viruses. Virology. 1971 Aug;45(2):356–363. doi: 10.1016/0042-6822(71)90336-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hunter T. R., Hunt T., Knowland J., Zimmern D. Messenger RNA for the coat protein of tobacco mosaic virus. Nature. 1976 Apr 29;260(5554):759–764. doi: 10.1038/260759a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Jackson A. O., Zaitlin M., Siegel A., Francki R. I. Replication of tobacco mosaic virus. 3. Viral RNA metabolism in separated leaf cells. Virology. 1972 Jun;48(3):655–665. doi: 10.1016/0042-6822(72)90150-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lebeurier G., Nicolaieff A., Richards K. E. Inside-out model for self-assembly of tobacco mosaic virus. Proc Natl Acad Sci U S A. 1977 Jan;74(1):149–153. doi: 10.1073/pnas.74.1.149. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Legrand M., Kauffmann S., Geoffroy P., Fritig B. Biological function of pathogenesis-related proteins: Four tobacco pathogenesis-related proteins are chitinases. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6750–6754. doi: 10.1073/pnas.84.19.6750. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Meshi T., Ishikawa M., Motoyoshi F., Semba K., Okada Y. In vitro transcription of infectious RNAs from full-length cDNAs of tobacco mosaic virus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5043–5047. doi: 10.1073/pnas.83.14.5043. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Namba K., Stubbs G. Structure of tobacco mosaic virus at 3.6 A resolution: implications for assembly. Science. 1986 Mar 21;231(4744):1401–1406. doi: 10.1126/science.3952490. [ DOI ] [ PubMed ] [ Google Scholar ]
- Ohno T., Takamatsu N., Meshi T., Okada Y., Nishiguchi M., Kiho Y. Single amino acid substitution in 30K protein of TMV defective in virus transport function. Virology. 1983 Nov;131(1):255–258. doi: 10.1016/0042-6822(83)90551-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- Osman T. A., Buck K. W. The tobacco mosaic virus RNA polymerase complex contains a plant protein related to the RNA-binding subunit of yeast eIF-3. J Virol. 1997 Aug;71(8):6075–6082. doi: 10.1128/jvi.71.8.6075-6082.1997. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pelham H. R. Leaky UAG termination codon in tobacco mosaic virus RNA. Nature. 1978 Mar 30;272(5652):469–471. doi: 10.1038/272469a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Register J. C., 3rd, Beachy R. N. Resistance to TMV in transgenic plants results from interference with an early event in infection. Virology. 1988 Oct;166(2):524–532. doi: 10.1016/0042-6822(88)90523-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- Saito T., Meshi T., Takamatsu N., Okada Y. Coat protein gene sequence of tobacco mosaic virus encodes a host response determinant. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6074–6077. doi: 10.1073/pnas.84.17.6074. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stanley W. M. ISOLATION OF A CRYSTALLINE PROTEIN POSSESSING THE PROPERTIES OF TOBACCO-MOSAIC VIRUS. Science. 1935 Jun 28;81(2113):644–645. doi: 10.1126/science.81.2113.644. [ DOI ] [ PubMed ] [ Google Scholar ]
- TSUGITA A. The proteins of mutants of TMV: composition and structure of chemically evoked mutants of TMV RNA. J Mol Biol. 1962 Sep;5:284–292. doi: 10.1016/s0022-2836(62)80072-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- Takamatsu N., Ishikawa M., Meshi T., Okada Y. Expression of bacterial chloramphenicol acetyltransferase gene in tobacco plants mediated by TMV-RNA. EMBO J. 1987 Feb;6(2):307–311. doi: 10.1002/j.1460-2075.1987.tb04755.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Takebe I., Otsuki Y. Infection of tobacco mesophyll protoplasts by tobacco mosaic virus. Proc Natl Acad Sci U S A. 1969 Nov;64(3):843–848. doi: 10.1073/pnas.64.3.843. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tsugita A., Gish D. T., Young J., Fraenkel-Conrat H., Knight C. A., Stanley W. M. THE COMPLETE AMINO ACID SEQUENCE OF THE PROTEIN OF TOBACCO MOSAIC VIRUS. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1463–1469. doi: 10.1073/pnas.46.11.1463. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- WATSON J. D. The structure of tobacco mosaic virus. I. X-ray evidence of a helical arrangement of sub-units around the longitudinal axis. Biochim Biophys Acta. 1954 Jan;13(1):10–19. doi: 10.1016/0006-3002(54)90265-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- Westhof E., Altschuh D., Moras D., Bloomer A. C., Mondragon A., Klug A., Van Regenmortel M. H. Correlation between segmental mobility and the location of antigenic determinants in proteins. Nature. 1984 Sep 13;311(5982):123–126. doi: 10.1038/311123a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Whitham S., Dinesh-Kumar S. P., Choi D., Hehl R., Corr C., Baker B. The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin-1 receptor. Cell. 1994 Sep 23;78(6):1101–1115. doi: 10.1016/0092-8674(94)90283-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wilson T. M. Plant viruses: a tool-box for genetic engineering and crop protection. Bioessays. 1989 Jun;10(6):179–186. doi: 10.1002/bies.950100602. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wolf S., Deom C. M., Beachy R. N., Lucas W. J. Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science. 1989 Oct 20;246(4928):377–379. doi: 10.1126/science.246.4928.377. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wu X., Shaw J. Bidirectional uncoating of the genomic RNA of a helical virus. Proc Natl Acad Sci U S A. 1996 Apr 2;93(7):2981–2984. doi: 10.1073/pnas.93.7.2981. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zimmern D. The nucleotide sequence at the origin for assembly on tobacco mosaic virus RNA. Cell. 1977 Jul;11(3):463–482. doi: 10.1016/0092-8674(77)90065-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- van Loon L. C., van Kammen A. Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. "Samsun" and "Samsun NN". II. Changes in protein constitution after infection with tobacco mosaic virus. Virology. 1970 Feb;40(2):190–211. doi: 10.1016/0042-6822(70)90395-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (193.3 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Tobacco Mosaic Virus: Pioneering Research for a Century
A n creager, k scholthof, h b scholthof.
- Copyright and License information
The Full Text of this article is available as a PDF (92.7 KB).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERER F. A., UHLIG H., WEBER E., SCHRAMM G. Primary structure of the protein of tobacco mosaic virus. Nature. 1960 Jun 18;186:922–925. doi: 10.1038/186922a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Abel P. P., Nelson R. S., De B., Hoffmann N., Rogers S. G., Fraley R. T., Beachy R. N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science. 1986 May 9;232(4751):738–743. doi: 10.1126/science.3457472. [ DOI ] [ PubMed ] [ Google Scholar ]
- Atabekov J. G., Taliansky M. E. Expression of a plant virus-coded transport function by different viral genomes. Adv Virus Res. 1990;38:201–248. doi: 10.1016/s0065-3527(08)60863-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- Atkinson P. H., Matthews R. E. On the origin of dark green tissue in tobacco leaves infected with tobacco mosaic virus. Virology. 1970 Feb;40(2):344–356. doi: 10.1016/0042-6822(70)90411-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- Beachy R. N., Zaitlin M., Bruening G., Israel H. W. A genetic map for the cowpea strain on TMV. Virology. 1976 Sep;73(2):498–507. doi: 10.1016/0042-6822(76)90411-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bendahmane M., Fitchen J. H., Zhang G., Beachy R. N. Studies of coat protein-mediated resistance to tobacco mosaic tobamovirus: correlation between assembly of mutant coat proteins and resistance. J Virol. 1997 Oct;71(10):7942–7950. doi: 10.1128/jvi.71.10.7942-7950.1997. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bos L. The embryonic beginning of virology: unbiased thinking and dogmatic stagnation. Arch Virol. 1995;140(3):613–619. doi: 10.1007/BF01718437. [ DOI ] [ PubMed ] [ Google Scholar ]
- Butler P. J. The current picture of the structure and assembly of tobacco mosaic virus. J Gen Virol. 1984 Feb;65(Pt 2):253–279. doi: 10.1099/0022-1317-65-2-253. [ DOI ] [ PubMed ] [ Google Scholar ]
- CASPAR D. L. The structural stability of tocacco mosaic virus. Trans N Y Acad Sci. 1960 May;22:519–521. doi: 10.1111/j.2164-0947.1960.tb00721.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- CRICK F. H., WATSON J. D. Structure of small viruses. Nature. 1956 Mar 10;177(4506):473–475. doi: 10.1038/177473a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Citovsky V., Knorr D., Schuster G., Zambryski P. The P30 movement protein of tobacco mosaic virus is a single-strand nucleic acid binding protein. Cell. 1990 Feb 23;60(4):637–647. doi: 10.1016/0092-8674(90)90667-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- Citovsky V., McLean B. G., Zupan J. R., Zambryski P. Phosphorylation of tobacco mosaic virus cell-to-cell movement protein by a developmentally regulated plant cell wall-associated protein kinase. Genes Dev. 1993 May;7(5):904–910. doi: 10.1101/gad.7.5.904. [ DOI ] [ PubMed ] [ Google Scholar ]
- Clark S. E. Organ Formation at the Vegetative Shoot Meristem. Plant Cell. 1997 Jul;9(7):1067–1076. doi: 10.1105/tpc.9.7.1067. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Clark S. E., Williams R. W., Meyerowitz E. M. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell. 1997 May 16;89(4):575–585. doi: 10.1016/s0092-8674(00)80239-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- Dawson W. O., Beck D. L., Knorr D. A., Grantham G. L. cDNA cloning of the complete genome of tobacco mosaic virus and production of infectious transcripts. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1832–1836. doi: 10.1073/pnas.83.6.1832. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Deom C. M., Oliver M. J., Beachy R. N. The 30-kilodalton gene product of tobacco mosaic virus potentiates virus movement. Science. 1987 Jul 24;237(4813):389–394. doi: 10.1126/science.237.4813.389. [ DOI ] [ PubMed ] [ Google Scholar ]
- FRAENKEL-CONRAT H., SINGER B. Virus reconstitution. II. Combination of protein and nucleic acid from different strains. Biochim Biophys Acta. 1957 Jun;24(3):540–548. doi: 10.1016/0006-3002(57)90244-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- FRANKLIN R. E. Structure of tobacco mosaic virus. Nature. 1955 Feb 26;175(4452):379–381. doi: 10.1038/175379a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Fraenkel-Conrat H., Williams R. C. RECONSTITUTION OF ACTIVE TOBACCO MOSAIC VIRUS FROM ITS INACTIVE PROTEIN AND NUCLEIC ACID COMPONENTS. Proc Natl Acad Sci U S A. 1955 Oct 15;41(10):690–698. doi: 10.1073/pnas.41.10.690. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fraile A., Escriu F., Aranda M. A., Malpica J. M., Gibbs A. J., García-Arenal F. A century of tobamovirus evolution in an Australian population of Nicotiana glauca. J Virol. 1997 Nov;71(11):8316–8320. doi: 10.1128/jvi.71.11.8316-8320.1997. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- GIERER A., MUNDRY K. W. Production of mutants of tobacco mosaic virus by chemical alteration of its ribonucleic acid in vitro. Nature. 1958 Nov 22;182(4647):1457–1458. doi: 10.1038/1821457a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- GIERER A., SCHRAMM G. Infectivity of ribonucleic acid from tobacco mosaic virus. Nature. 1956 Apr 14;177(4511):702–703. doi: 10.1038/177702a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gafny R., Lapidot M., Berna A., Holt C. A., Deom C. M., Beachy R. N. Effects of terminal deletion mutations on function of the movement protein of tobacco mosaic virus. Virology. 1992 Apr;187(2):499–507. doi: 10.1016/0042-6822(92)90452-u. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gallie D. R., Sleat D. E., Watts J. W., Turner P. C., Wilson T. M. The 5'-leader sequence of tobacco mosaic virus RNA enhances the expression of foreign gene transcripts in vitro and in vivo. Nucleic Acids Res. 1987 Apr 24;15(8):3257–3273. doi: 10.1093/nar/15.8.3257. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Heinlein M., Epel B. L., Padgett H. S., Beachy R. N. Interaction of tobamovirus movement proteins with the plant cytoskeleton. Science. 1995 Dec 22;270(5244):1983–1985. doi: 10.1126/science.270.5244.1983. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hua J., Sakai H., Nourizadeh S., Chen Q. G., Bleecker A. B., Ecker J. R., Meyerowitz E. M. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell. 1998 Aug;10(8):1321–1332. doi: 10.1105/tpc.10.8.1321. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hunter T. R., Hunt T., Knowland J., Zimmern D. Messenger RNA for the coat protein of tobacco mosaic virus. Nature. 1976 Apr 29;260(5554):759–764. doi: 10.1038/260759a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Jackson A. O., Zaitlin M., Siegel A., Francki R. I. Replication of tobacco mosaic virus. 3. Viral RNA metabolism in separated leaf cells. Virology. 1972 Jun;48(3):655–665. doi: 10.1016/0042-6822(72)90150-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kay L. E. A book of life? How the genome became an information system and DNA a language. Perspect Biol Med. 1998 Summer;41(4):504–528. doi: 10.1353/pbm.1998.0038. [ DOI ] [ PubMed ] [ Google Scholar ]
- LAUFFER M. A., ANSEVIN A. T., CARTWRIGHT T. E., BRINTON C. C., Jr Polymerization-depolymerization of tobacco mosaic virus protein. Nature. 1958 May 10;181(4619):1338–1339. doi: 10.1038/1811338b0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lartey R. T., Ghoshroy S., Citovsky V. Identification of an Arabidopsis thaliana mutation (vsm1) that restricts systemic movement of tobamoviruses. Mol Plant Microbe Interact. 1998 Jul;11(7):706–709. doi: 10.1094/MPMI.1998.11.7.706. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lease K., Ingham E., Walker J. C. Challenges in understanding RLK function. Curr Opin Plant Biol. 1998 Oct;1(5):388–392. doi: 10.1016/s1369-5266(98)80261-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- McLean B. G., Zupan J., Zambryski P. C. Tobacco mosaic virus movement protein associates with the cytoskeleton in tobacco cells. Plant Cell. 1995 Dec;7(12):2101–2114. doi: 10.1105/tpc.7.12.2101. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Meshi T., Ishikawa M., Motoyoshi F., Semba K., Okada Y. In vitro transcription of infectious RNAs from full-length cDNAs of tobacco mosaic virus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5043–5047. doi: 10.1073/pnas.83.14.5043. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Namba K., Pattanayek R., Stubbs G. Visualization of protein-nucleic acid interactions in a virus. Refined structure of intact tobacco mosaic virus at 2.9 A resolution by X-ray fiber diffraction. J Mol Biol. 1989 Jul 20;208(2):307–325. doi: 10.1016/0022-2836(89)90391-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- Ohno T., Takamatsu N., Meshi T., Okada Y., Nishiguchi M., Kiho Y. Single amino acid substitution in 30K protein of TMV defective in virus transport function. Virology. 1983 Nov;131(1):255–258. doi: 10.1016/0042-6822(83)90551-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- Pelham H. R. Leaky UAG termination codon in tobacco mosaic virus RNA. Nature. 1978 Mar 30;272(5652):469–471. doi: 10.1038/272469a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- Solano R., Ecker J. R. Ethylene gas: perception, signaling and response. Curr Opin Plant Biol. 1998 Oct;1(5):393–398. doi: 10.1016/s1369-5266(98)80262-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- Stanley W. M. ISOLATION OF A CRYSTALLINE PROTEIN POSSESSING THE PROPERTIES OF TOBACCO-MOSAIC VIRUS. Science. 1935 Jun 28;81(2113):644–645. doi: 10.1126/science.81.2113.644. [ DOI ] [ PubMed ] [ Google Scholar ]
- Stone J. M., Collinge M. A., Smith R. D., Horn M. A., Walker J. C. Interaction of a protein phosphatase with an Arabidopsis serine-threonine receptor kinase. Science. 1994 Nov 4;266(5186):793–795. doi: 10.1126/science.7973632. [ DOI ] [ PubMed ] [ Google Scholar ]
- Stone JM, Trotochaud AE, Walker JC, Clark SE. Control of meristem development by CLAVATA1 receptor kinase and kinase-associated protein phosphatase interactions . Plant Physiol. 1998 Aug;117(4):1217–1225. doi: 10.1104/pp.117.4.1217. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tari A. M., Hung M. C., Li K., Lopez-Berestein G. Growth inhibition of breast cancer cells by Grb2 downregulation is correlated with inactivation of mitogen-activated protein kinase in EGFR, but not in ErbB2, cells. Oncogene. 1999 Feb 11;18(6):1325–1332. doi: 10.1038/sj.onc.1202422. [ DOI ] [ PubMed ] [ Google Scholar ]
- Trotochaud A. E., Hao T., Wu G., Yang Z., Clark S. E. The CLAVATA1 receptor-like kinase requires CLAVATA3 for its assembly into a signaling complex that includes KAPP and a Rho-related protein. Plant Cell. 1999 Mar;11(3):393–406. doi: 10.1105/tpc.11.3.393. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tsugita A., Gish D. T., Young J., Fraenkel-Conrat H., Knight C. A., Stanley W. M. THE COMPLETE AMINO ACID SEQUENCE OF THE PROTEIN OF TOBACCO MOSAIC VIRUS. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1463–1469. doi: 10.1073/pnas.46.11.1463. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- WATSON J. D. The structure of tobacco mosaic virus. I. X-ray evidence of a helical arrangement of sub-units around the longitudinal axis. Biochim Biophys Acta. 1954 Jan;13(1):10–19. doi: 10.1016/0006-3002(54)90265-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- Waigmann E., Lucas W. J., Citovsky V., Zambryski P. Direct functional assay for tobacco mosaic virus cell-to-cell movement protein and identification of a domain involved in increasing plasmodesmal permeability. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1433–1437. doi: 10.1073/pnas.91.4.1433. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Whitham S., McCormick S., Baker B. The N gene of tobacco confers resistance to tobacco mosaic virus in transgenic tomato. Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8776–8781. doi: 10.1073/pnas.93.16.8776. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Williams R. W., Wilson J. M., Meyerowitz E. M. A possible role for kinase-associated protein phosphatase in the Arabidopsis CLAVATA1 signaling pathway. Proc Natl Acad Sci U S A. 1997 Sep 16;94(19):10467–10472. doi: 10.1073/pnas.94.19.10467. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wolf S., Deom C. M., Beachy R. N., Lucas W. J. Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science. 1989 Oct 20;246(4928):377–379. doi: 10.1126/science.246.4928.377. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wu X., Shaw J. G. Evidence that a viral replicase protein is involved in the disassembly of tobacco mosaic virus particles in vivo. Virology. 1997 Dec 22;239(2):426–434. doi: 10.1006/viro.1997.8870. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wu X., Shaw J. Bidirectional uncoating of the genomic RNA of a helical virus. Proc Natl Acad Sci U S A. 1996 Apr 2;93(7):2981–2984. doi: 10.1073/pnas.93.7.2981. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (92.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Practicing virology: making and knowing a mid-twentieth century experiment with Tobacco mosaic virus
- Original Paper
- Open access
- Published: 01 February 2022
- Volume 44 , article number 3 , ( 2022 )
Cite this article
You have full access to this open access article

- Karen-Beth G. Scholthof ORCID: orcid.org/0000-0002-8169-9823 1 ,
- Lorenzo J. Washington ORCID: orcid.org/0000-0002-0624-4693 nAff2 ,
- April DeMell ORCID: orcid.org/0000-0001-8345-075X nAff3 ,
- Maria R. Mendoza nAff4 &
- Will B. Cody ORCID: orcid.org/0000-0002-8894-5580 nAff5
5264 Accesses
5 Citations
6 Altmetric
Explore all metrics
Tobacco mosaic virus (TMV) has served as a model organism for pathbreaking work in plant pathology, virology, biochemistry and applied genetics for more than a century. We were intrigued by a photograph published in Phytopathology in 1934 showing that Tabasco pepper plants responded to TMV infection with localized necrotic lesions, followed by abscission of the inoculated leaves. This dramatic outcome of a biological response to infection observed by Francis O. Holmes, a virologist at the Rockefeller Institute for Medical Research, was used to score plants for resistance to TMV infection. Our objective was to gain a better understanding of early to mid-twentieth century ideas of genetic resistance to viruses in crop plants. We investigated Holmes’ observation as a practical exercise in reworking an experiment, having been inspired by Pamela Smith’s innovative Making and Knowing Project. We had a great deal of difficulty replicating Holmes’ experiment, finding that biological materials and experimental customs change over time, in ways that ideas do not. Using complementary tools plus careful study and interpretation of the original text and figures, we were able to rework, yet only partially replicate, this experiment. Reading peer-reviewed manuscripts that cited Holmes’ 1934 report provided an additional level of insight into the interpretation and replication of this work in the decades that followed. From this, we touch on how experimental reworking can inform our strategies to address the reproducibility “crisis” in twenty-first century science.
Similar content being viewed by others

Plant Viruses: Factors Involved in Emergence and Recent Advances in Their Management

Emerging strategies in plant virus disease control: insights from the 56th meeting of the DPG working group “Viruskrankheiten der Pflanzen”


Masters of manipulation: how do positive-sense RNA viruses employ plant proteins to replicate, move from cell to cell, and overcome antiviral immunity?
Avoid common mistakes on your manuscript.
If a photograph is worth a thousand words, then we were taken (in) by an image from a 1934 scientific manuscript in the journal Phytopathology (Fig. 1 ). The figure shows a Tabasco pepper leaf dropping from the plant following inoculation with Tobacco mosaic virus (TMV). Tabasco plants respond to TMV infection within a few days of inoculation, first with localized necrotic lesions (LNLs) on the inoculated leaf. The LNLs are mere pinpoints, oftentimes all but obscured by the damage incurred by rub-inoculation. Leaf abscission occurs a few days after LNLs are observed. This response—to sacrifice an inoculated leaf to rid itself of the virus—is a dramatic outcome. Francis O. Holmes, a virologist at the Rockefeller Institute for Medical Research, used both responses to monitor for the presence of a dominant gene for resistance to TMV infection. Footnote 1

Photograph showing the effects of TMV-infection on homozygous ( ll ) and heterozygous ( LL of Ll) plants from the genetic cross of Tabasco X bell pepper. The figure caption reads: “Two plants of Capsicum frutescens , inoculated with tobacco-mosaic virus. The first was a mottling-type plant and the second, a necrotic-type. A. 3 days after inoculation of 2 leaves each. B. 7 days after inoculation. Inoculated leaves had fallen from necrotic-type plant, freeing it from virus [arrow added for emphasis]. C. 16 days after inoculation. Mottling-type plant was stunted and mottled. Necrotic-type one was large, without symptoms, and free of virus” (Holmes, 1934 , p. 988). The notations of symptom type, mottling ( ll ) and localized necrotic lesions ( Ll and LL plants); and the days post-inoculation (dpi) with TMV were added to clarify Holmes’ experimental results. (Holmes, 1934 , Fig. 2 , p. 988, used with permission of the American Phytopathology Society.)
We were interested in replicating this experiment as an exemplar of “practicing virology” within the context of the history of science. Our work, initially inspired by Pamela Smith’s Making and Knowing Project, was fraught with challenges. A seemingly simple experiment belied the complexity and challenges of reworking an experiment from the past. We concluded that some experiments from the past cannot be replicated in full; that complementary methods are oftentimes necessary to interpret experimental results across the decades; that careful and attentive reading and interpretation of text and figures is necessary and essential to rework an experiment; and, identification and reading manuscripts that cite the original work is an extremely useful tool to interpretate historical experiments. Here we discuss our challenges and successes with reference to findings from historians of science who have reworked interesting experiments of the past. We also touch on the role of craft (making) and pitfalls associated with biological materials for historical reworking (knowing). In relation to a perceived “reproducibility crisis” in recent science, we discuss, in light of our experience, potential difficulties in reworking experiments, which includes identifying, replicating, funding and publishing the results. Finally, we hope our experiments will encourage more hands-on reworking as a key component of the historiography of the life sciences because of its informative value.
1 Practicing virology
Prior to the rediscovery and wide-spread acceptance of Mendelian genetics, crop improvement was based on observation. Plant pathologists and breeders would survey fields, collecting seeds of plants with desirable traits, such as improved yield, or escape from the ravages of diseases. This seed would be increased and used in subsequent seasons. Another, more focused strategy, evaluated seed from local collections or that provided by the USDA. Footnote 2 With Mendelian genetics, plant breeders in the twentieth century could deliberately introduce new, desirable traits to crop plants. Such “inheritable traits could be charted through mathematical probabilities” allowing for “efficient and predictable” outcomes including genetic resistance to plant pathogens (Campbell et al., 1999 , p. 257). Seeds were harvested from plants with the desired phenotypes, followed by pathogen challenge of a new generation of (hybrid) plants. Plants tolerant or resistant to the challenge were advanced through the trials, grown to maturity and their seed harvested. Plants from these seeds, were backcrossed to plants with commercially desirable features. A stable genetic line would be developed with nearly all the original “good” features of a parent plant with the addition of genetic resistance to a particular pathogen. This work could take years. (While todays molecular methods allow for more rapid identification of the resistance genes, the breeding process remains labor and time intensive.) Finally, the seed would be increased for commercial use. Footnote 3
Tobacco mosaic virus was one pathogen causing economic losses in tobacco, pepper and tomato fields. In the early twentieth century, understanding the “nature” of the virus was an enormously difficult task as viruses could not be cultured or observed by light microscopy. By necessity, indirect methods were developed to study viruses and their interactions with host plants (Fig. 2 ). Francis O. Holmes was a scientist who is now recognized for creating innovative and reproducible advances in virology and plant breeding in the early twentieth century, first at the Boyce Thompson Institute for Plant Research (Yonkers, NY), then the Rockefeller Institute for Medical Research (Princeton, NJ). He reported on the development of a biological assay for plant viruses that involved the visualization of TMV infection on tobacco and other plants (Holmes, 1929b ) (Fig. 2 ). Holmes observed small LNLs accumulating on TMV-inoculated Nicotiana glutinosa leaves. The virus was confined within the boundaries of the lesions on the inoculated leaf—this host response was protective, allowing the tobacco plant to complete its lifecycle without detriment. Holmes determined this response was due to N. glutinosa harboring a single dominant gene ( N ) for resistance to TMV infection (Holmes, 1929b , 1931 ; Scholthof, 2004 , 2011 , 2014 ). Then, he used Mendelian techniques to cross the N. glutinosa gene- N into N. tabacum (tobacco) as a first step to develop commercial tobacco lines with field resistance to TMV (Holmes, 1934 , 1938 ; Scholthof, 2014 , 2016 ). Footnote 4 The LNL response to TMV infection was used as a biological assay to confirm the introgression of the N -gene into tobacco plants.

Illustration of mechanical inoculation of plant viruses as shown in the 2nd edition of Plant Pathology , a textbook by George N. Agrios ( 1978 ) used by generations of plant pathologists. (Agrios, 1978 , Fig. 213, p. 568, used with permission of Elsevier.)
This process was fraught with difficulties in that it took Holmes three years to advance this project (Holmes, 1934 , 1938 ; Scholthof, 2014 , 2016 ). During this experimental interregnum Holmes pursued a similar approach with pepper ( Capsicum species), finding it more amenable to Mendelian breeding strategies. Footnote 5 He had determined that Tabasco pepper leaves inoculated with TMV developed small LNLs, then dropped from the plant a few days later (Fig. 1 ), rendering the plant virus free. Holmes attributed this effect to the presence of the Tabasco gene L , analogous to the N. glutinosa N -gene. With this knowledge, Holmes incorporated the Tabasco gene L into commercial lines of bell pepper, thus protecting the plants from systemic TMV infection. Footnote 6 Today, this same L -gene is found in TMV-resistant bell pepper cultivars. The 1934 publication is important to plant pathology because it was the first demonstration that a resistance gene from one species could be used to protect another species from the ravages of virus infection. As shown by Holmes, Tabasco pepper leaves abscised within days of TMV inoculation, a striking means to visualize a gene-in-action in the pre-molecular biology era.
The manifest issues of technique, skill, tools, and temporal distance have been addressed by Pamela Smith’s pathbreaking “Making and Knowing Project” at Columbia University. Smith has commented on “how odd it is that historians whose object of study is historical materials and techniques … have generally not considered engagement with the materials of their historical topics as an essential part of their training and research” (Smith, 2016 , p. 9). Here, acting as scientists and practitioner-historians, we investigate a historical topic and the value of tacit (or gestural) knowledge in experiment and interpretation. We concur with Smith that making and knowing is an “necessary part of our intellectual toolbox ... through hands-on work with materials and techniques,” and that the devil is in the details—some of which, as we will show, are details that we had initially not considered (Smith, 2016 , p. 9). We explore, through demonstration, the complexity of “doing biology” across the decades. We found that although ideas travel, the biological components (plants, viruses) and performance of a technique are more difficult to locate. Footnote 7
In his TEA set paper Harry Collins addressed the difficulty of replication across physical distance, even for those expert in their area of practice and craft (Collins, 1974 ). Footnote 8 Collins interviewed TEA laser scientists, finding that peer-reviewed publications and citations were used to suggest “the flow of articulated and therefore visible information,” but this did not give a full understanding of “the modes of transfer of real, useable knowledge among a set of scientists” (Collins, 1974 , pp. 170, 174). We are attempting to develop and construct parameters to transfer information—an experiment (Fig. 1 ). For Collins, the trial and error aspects of developing a new technology (tool) and “the non-systematic element” (Collins, 1974 , p. 175) were part of the process of making and knowing, something we also encountered in setting up this “simple” TMV experiment.
Here, we provide an example of reproducing knowledge at a temporal distance, using a biological experiment. We encountered many of the same problems mentioned in the TEA set paper as we worked to reproduce an experiment from a written document. Collins indicated that “written sources … as the sole source of information” are inadequate and the ability to reproduce an experiment or build a piece of equipment or “reinvent it” indicates that the (naïve) group “knows as much” as the reporter (Collins, 1974 , p. 176). Pamela Smith pulls these ideas together in a material framework (Smith, 2012 ). The “how to” comes about with deliberate reading, interpretation, testing, experimentation, and analysis of the results. In all instances repetition is key to mastering each step in the reworking—the craft of becoming a “maker”. Interpretation, analysis and extension of the findings is “knowing”. This iterative process entails significant time, material resources, hands-on experience, mistakes, troubleshooting, and critical thinking.
Several scholars have been at the forefront in engaging in the “experimental history of science” (Fors et al., 2016 , p. 89) to deepen our understanding of the insight, craft, practice, and ideas of early physical and chemical scientists (Ahnfelt & Fors, 2016 ; Ahnfelt et al., 2020 ; Albala, 2010 ; Barwich & Rodriguez, 2020 ; Bilak, 2020 ; Chang, 2011 ; Fors et al., 2016 ; Hendriksen, 2020 ; Hendriksen & Verwaal, 2020 ; Principe, 1987 ; Root-Bernstein, 1983 ; Sibum, 1995 ; Smith, 2012 ; Usselman et al., 2005 ). Their contextualization of the historiography through experimentation brings us a richer understanding of scientific processes, development, and epistemology. Yet, little reworking has occurred within the life sciences.
One example of biological reworking was a counting study by Robert Root-Bernstein. This project revealed the difficulty of reproducing a seemingly straightforward problem in biology: identification by observation of seed characteristics (the phenotype) using maize kernels (Root-Bernstein, 1983 ). As described by Root-Bernstein, the early twentieth century controversy surrounding the results of Mendel’s garden pea study (when 1936 Ronald Fisher proclaimed that the counting must be off or that some fudging occurred because it surely was not possible to have those precise predicted ratios) could be resolved by a simple experiment. Instead of using peas, Root-Bernstein selected maize, using a monohybrid cross (pure lines of purple seed X yellow seed parents); the hybrid would produce, according to Mendel, an equal ratio of purple:yellow kernels. He asked undergraduate students to count the number of purple or yellow kernels on an ear. Root-Bernstein found that it is more difficult to assess a phenotype (the physical expression of a gene) than expected, with upwards of 2% of the kernels “indeterminant” or “difficult to classify”. However, the general results were in line with what was predicted by Mendelian ratios. A more difficult task of scoring two dihybrid crosses with the “traits purple, yellow, wrinkled and smooth,” classified 6% of the kernels as “indeterminate” (Root-Bernstein, 1983 , p. 284).
Root-Bernstein’s work leads us to a similar experiment reported by Raymond Pearl in 1911 (Pearl, 1911 ). Pearl used “fifteen trained observers” who “were required to discriminate only with reference to the color [yellow or white] and the form [starchy (smooth) or sweet (wrinkled)] of each kernel” with the expected Mendelian second generation ratios of 9 yellow starchy:3 yellow sweet:3 white starchy:1 white sweet (Pearl, 1930 , p. 127). Footnote 9 All observers counted 532 kernels, yet none of the “highly trained and competent observers” were in agreement concerning the distribution of the characteristics (Pearl, 1930 , p. 129). Pearl wrote that this “seems a simple problem. One only has to count them. They [the kernels] do not run away or change” (Pearl, 1930 , p. 129). This was a reworking at the most simple state – no preparation of plants, chemicals, inoculation, or cultivation. Merely counting. Root-Bernstein found that with practice, the students became better at making choices and in which bin to place the kernels. This outcome reminds us of the comment by Barbara McClintock to Evelyn Fox Keller that there is “a feeling for the organism” or, something that develops over time, allowing the experimentalist to ‘see’ and ‘understand’ more deeply with immersion than as a novice (Keller, 1984 ). We suggest that this is the beauty of reworking experiments. Making and knowing allows us to understand more about the methods and conclusions reported on by historical actors, the constraints associated with materials available in a given time period, and the experiential skills needed to accomplish fundamental, interesting studies in the sciences. The TMV-pepper experiment seemed an ideal project to learn more about Holmes’ ideas and his standard practices.
2 Making: materials and methods
The impetus for this experiment was the dramatic image of pepper leaf abscission several days following the LNL response to TMV inoculation, as shown by Holmes (Holmes, 1934 ) (Fig. 1 ). Our objective was to gain a better understanding of early to mid-twentieth century ideas of genetic resistance to viruses in crop plants. Footnote 10 Identifying the working biological materials (TMV strains and pepper plants) used by Holmes for his experiments was non-trivial. With only the briefest textual description of his methods in his published scientific papers, we had to interpret the experimental design.
2.1 Plants and planting
We purchased Tabasco and Heirloom California Wonder (sweet bell pepper) seeds from W. Atlee Burpee & Co. Two TMV-susceptible tobacco lines, N. tabacum cv. Turk and N. benthamiana (commonly used for laboratory experiments), were cultivated from our laboratory seed stock. Footnote 11 All plants were grown using the conditions shown in Table 1 . Footnote 12
Commercially produced seed introduces additional genetic variables, although the plants may appear to be identical (phenotype). For example, a genetic analysis of ten lines of California Wonder showed the plants could be grouped into 5 classes, based on genetic polymorphisms identified by PCR amplification with a series of primer sets to randomly sample the genome (Votava & Bosland, 2002 ). The authors cautioned that California Wonder “exists in name only” and its utility as a standard control should be determined based on the type of experiments performed (Votava & Bosland, 2002 , p. 1101). Footnote 13 Similarly, for Tabasco ( C. frutescens ) it is not possible to definitively state that the plant is identical to Holmes’ Tabasco; almost certainly it is not the same. Footnote 14 However, from observations made by Walter H. Greenleaf, a plant breeder and pathologist at Auburn University (Alabama), we know that “the L -gene in peppers provides an effective form of resistance” to “all tested strains of TMV from tobacco and tomato” (W. H. Greenleaf, 1986a , 1986b , p. 98), giving us a degree of confidence that a commercially available of Tabasco would be suitable for these experiments.
2.2 Rub inoculation
In the late 1920s, in a series of experiments, Holmes developed the rapid and efficient inoculation technique, now a standard practice, known as mechanical (or rub) inoculation (Holmes, 1928 , 1929a , 1929b , 1931 ) (Fig. 2 ). For rub-inoculation, one or more TMV-infected symptomatic N. tabacum leaves were pulverized with the addition of water or phosphate buffer (1:10 w/v), using a mortar and pestle. The negative control experiment, or mock-inoculation utilizes healthy leaves. Two or three lower leaves of a plant are rubbed gently with the sap extract following dusting with an abrasive powder (carborundum or Celite) to slightly injury the leaf, allowing virus ingress (Kalmus & Kassanis, 1945 ). Immediately after inoculation the leaves were rinsed with water. The plants were observed every day and symptoms were recorded, with particular attention to local lesions and systemic infections. Footnote 15
2.3 Tobacco mosaic virus (TMV)
The TMV common strain (U1) was maintained on N. tabacum cv. Turk and N. benthamiana . This strain induces necrotic local lesions on N. glutinosa and Tabasco pepper. Pepper leaves were rub-inoculated following Holmes’ method (Fig. 2 ). Unfortunately, due to rub inoculation damage on our plants, it was difficult to count lesions and to determine the level of infection. To rework this experiment we used a more tractable tool, an infectious TMV cDNA construct. This is a routine plant molecular virology practice to determine if pepper plants were susceptible to TMV infection. Footnote 16 Specifically, our complementary experiment utilized a molecular construct of TMV with the addition of the green fluorescent protein ( gfp ) gene (TMV-GFP) (Fig. 3 A). TMV-GFP infected tobacco leaves were harvested and used as inoculum for the pepper experiments (Fig. 2 ). Footnote 17 TMV-GFP was used to i) monitor virus infection (count local lesions) by fluorescence under ultraviolet light and ii) determine the sites of virus replication versus inoculation damage. TMV-GFP provided consistent and genetically homogeneous inoculum to investigate TMV infection, development of LNLs, and leaf abscission.

Exploring Holmes’ results with Tobacco mosaic virus (TMV) and pepper plants with the techniques of molecular biology. a The molecular genetic map of TMV with the addition of a reporter gene encoding the green fluorescent protein (GFP). The rectangles indicate protein-encoding genes of TMV: replicase, movement protein (MP), and capsid protein (CP). The bent arrows indicate the subgenomic RNA promoters. The asterisk indicates that a specialized strategy of readthrough translation to express two replicase proteins from the genomic RNA. b and c Representative Tabasco pepper ( Capsicum frutescens ) and Nicotiana tabacum cv. Turk (tobacco) leaves at 2, 3 and 4 days post-inoculation with TMV-GFP. The leaves were photographed under white light and ultraviolet (UV) light. In Fig. 3B, rub-inoculation damage of the inoculated leaves presents as brown discoloration under white light and greyish-white discoloration under UV light. The same leaves were used for white and UV light exposure. The localized green fluorescent spots on Tabasco and tobacco leaves reflect single infection events following inoculation with TMV-GPF, equivalent to the localized necrotic lesions reported by Holmes. On tobacco, the pinpoint florescence spots at 2 dpi become much larger by 4 dpi, indicating TMV resistance gene N is not present. In time these green fluorescent spots coalesce and progress to systemic infection (not shown). D. California Wonder bell pepper ( C. annuum ) plants showing systemic infection at 24 days post-inoculation with TMV-GFP
Other historians of science who had pursued their own reworking of experiments, reported using modern tools as they developed their craft (Ahnfelt & Fors, 2016 ; Ahnfelt et al., 2020 ; Albala, 2010 ; Barwich & Rodriguez, 2020 ; Bilak, 2020 ; Chang, 2011 ; Fors et al., 2016 ; Hendriksen & Verwaal, 2020 ; Principe, 1987 ; Root-Bernstein, 1983 ; Sibum, 1995 ; Usselman et al., 2005 ). For example, Hasok Chang uses modern instruments to understand historical experiments. For his “complementary” experiments on the boiling point of water, he explained “when practitioners of historical replication say they try to get ‘as close to the original as possible’, that is usually with a clear awareness of some inherent limits to faithfulness. It is not always possible to match exactly the past instruments and operations described in historical papers” (Chang, 2011 , p. 320). Chang also notes the historic manuscript may exclude some methodology because it was “well-understood by readers in the original context” (Chang, 2011 , p. 320). For these reasons, we introduced complementary molecular virology tools as “ opportunities for better historical understanding” (Chang, 2011 , p. 321).
3 Knowing: results
We rub-inoculated two or three leaves of small Tabasco and bell pepper plants with sap of TMV or mock-inoculated plants for the control experiment. Our expectation was that visible chlorotic lesions would develop on California Wonder bell pepper leaves within 7 to 10 days, followed by mottling of the upper, non-inoculated leaves. For Tabasco pepper, we expected to observe LNLs within a few days, followed by leaf abscission. Instead, in our hands, abscission was observed for mock- and TMV-inoculated bell pepper and Tabasco plants.
We were especially confounded by the abscission response we observed on mock-inoculated leaves. Holmes consistently emphasized that rapid leaf drop was a marker for the Tabasco L -gene. For example, TMV-infected L -gene segregating bell pepper lines, such as California Wonder, “show necrotic primary lesions only, and their inoculated leaves were soon lost by abscission” (Holmes, 1937 , p. 641). Footnote 18 However, Holmes also reported that older plants as well as plants maintained under different environmental conditions may defoliate independent of L -gene-associated abscission (Holmes, 1932 , p. 352). Altogether, we decided to focus our attention on (1) environmental conditions; (2) inoculation techniques, (3) confirming the mock-inoculated plants were not contaminated with TMV; and (4) the possibility of genetic variability of California Wonder, such as the inclusion of the L -gene or minor resistance genes or inadvertent contamination of seed lots.
As we know from Holmes, California Wonder is susceptible to TMV infection (Table 2 ). Footnote 19 Yet genetic variability within California Wonder occurs, as reported by Eric Votova and Paul Bosland, pepper breeders at New Mexico State University. This variability is a result of inadvertent mixing of seed lots by producers, intentional selection by plant breeders over time, or genetic drift (Votava & Bosland, 2002 ). Of course, it is impossible to rework the experiment with the exact same seeds Holmes used, which may have affected our interpretation of his findings (Table 2 ). However, we did determine that California Wonder was susceptible to TMV (Fig. 3 D). We then narrowed our considerations to environmental conditions and plant age.
Early on we observed leaf drop in almost all peppers—this was particularly evident when there were changes in the environment, including decreased temperatures in the growth chamber, or lab, due to power failures or maintenance issues, or biological contamination of the growth chambers with insects and fungi (a complication of working in shared spaces in a plant pathology department). Our first estimation of plant age was based on plant height and the approximate leaf size (Fig. 1 ; 4-inch diameter clay pots). We returned to Fig. 1 and determined that Holmes had used more mature plants, based on a count of the visible internodes. From this, we decided it would be worthwhile to test older plants for the abscission response.
4 Doing it again: laboratory practice and practicing
Plant virus inoculation and the molecular biology technique of the plasmid prep (isolating plasmid DNA from bacteria, generally E. coli ), are both considered straightforward “ubiquitous practice” (Jordan & Lynch, 1992 , p. 78). These methods of practice are so basic that they are used in undergraduate laboratory exercises (Dijkstra & De Jager, 1998 ; Ford & Evans, 2003 ). As elaborated by Kathleen Jordan and Michael Lynch, seemingly rote processes are predicated on more than the ability to read a protocol. Oftentimes there are “persistent problems associated with establishing the coherence and efficacy of the practice, determining whether one practitioner’s method for doing it is the same as another’s, accounting for discrepant results, and explaining how the technique works” (Jordan & Lynch, 1992 , p. 77). Importantly, this is in spite of the protocol being “relatively standardized, reproducible, coherent, and subject to rational reconstruction” (Jordan & Lynch, 1992 , p. 77). Yet protocols are neither rational or standardized without technique—typically acquired through apprenticeship. Here we are evaluating two aspects of a “mundane practice” (Jordan & Lynch, 1992 , p. 78): i) are Holmes’ observations reproducible in our hands? And ii) what sort of expertise matters to recapitulate previously published data?
In Jordan and Lynch’s study, they interrogated practitioners to learn about differences in a common practice, asking about variation “between their own and others’ methods” as well as “local circumstances of the lab and idiosyncrasies of its members” (Jordan & Lynch, 1992 , p. 78). Like the plasmid prep, virus inoculation is a key, mundane practice that must be learned (Figs. 1 , 2 and 3 ). Pamela Smith and Tonny Beentjes discuss this “makers’ knowledge” within the context of reconstructing life-casting techniques in the sixteenth-century. They emphasized that the “knowledge possessed by handworkers, also known as ‘makers’ knowledge’” is key to understanding the materials, techniques and “how and why nature was investigated” (Smith & Beentjes, 2010 , p. 130).
The “simplicity” of TMV inoculation of tobacco is made evident by its common use as an experiential tool for in plant pathology laboratory courses (Dijkstra & De Jager, 1998 ; Ford & Evans, 2003 ). Yet, rub inoculation is a particular practice subject to many errors, including damaging plants by rubbing leaves with too much enthusiasm (Fig. 3 A). The experimental outcome “can depend on the particular ingredients used, as well as an endless array of other circumstantial features” (Jordan & Lynch, 1992 , p. 81), even for a virus inoculation method standardized in the mid-1930s. Footnote 20
We systemically compared our materials and methods to those reported by Holmes (Table 1 ) and identified many variables, some of which may have affected the outcome of our reworking experiments. For example, in our hands pepper was exquisitely sensitive to environmental conditions, especially changes in ambient temperature. When we returned to the text, making a more careful study of his publications we found that Holmes had reported that TMV-susceptible Capsicum (and several other plant species) exposed to cooler growing temperatures may experience premature leaf abscission (Holmes, 1932 , p. 337). Another identified variable was plant age. When carefully inspecting Fig. 1 , we noticed that Holmes’ plants had several internodes, indicating more mature plants. In our subsequent reworking experiments, we used older plants. But our plant growth conditions resulted in tall plants with with elongated internodes, a result of low light intensity (Fig. 4 ). The variables that had foiled our initial efforts encompassed the key determinants of infection: the host, the virus, and the environment. Parsing the most important variables towards becoming proficient with Holmes’ methods, we realized the experimental protocol had features that were strikingly similar to those mentioned by Jordan and Lynch: “Although the plasmid prep is far from controversial and is commonly referenced as a well-established and indispensable technique, how exactly it is done is not effectively communicated, either by print, word of mouth or demonstration. Instead, it is mastered largely through repeated (and often solitary) practice” (Jordan & Lynch, 1992 , p. 84).

Recapitulation of the Tabasco pepper experiments described by Holmes. Panels a , b , and c . Wildtype TMV inoculated to Tabasco, as shown in Fig. 1 , and photographed at 3, 7 and 15 days postinoculation (dpi). On Tabasco leaves the necrotic pinpoint local lesions are difficult to observe, especially when the leaves are damaged during inoculation. b and c The TMV-inoculated leaf abscission noted at 7- and 15-dpi on two plants; mock inoculated leaves at 7 dpi have not abscised. An “X” on the leaf indicates that the leaf was inoculated (TMV or mock). In B, the center figure is a close up of the dropped leaf shown in the leftmost photograph. These results can be compared to those Holmes ( 1934 ), shown in Fig. 1
Initially this seemed a straightforward project to gain some understanding about how Holmes performed his experiments and if we could achieve similar results. What we know now is that these seemingly trivial experiments were fraught with technical difficulty and a great deal of complexity, even though we were merely pulverizing a TMV-infected leaf, rubbing it on a healthy pepper leaf and observing the outcomes of infection (mottling, leaf drop, etc.). The choices we and Holmes made were not trivial or insignificant. As discussed by Jordan and Lynch in their analysis of the standardized protocol for plasmid preps: “For practitioners at the bench, these distinctions [choices] do not easy resolve such issues as what to include or exclude from a procedure, what to do now, and what to do next” (Jordan & Lynch, 1992 , p. 100). We were handicapped by the lack of detailed protocols and an ever increasing number of parameters to attend to. Upon reflection, the lack of detailed materials and methods is not a Holmes-specific issue, nor is it an issue limited to historical biological reworking.
5 Reading between the lines: reproducibility
Our difficulty in reworking a decades-old experiment gets at broader questions. Is it possible to reproduce an experiment? Is an experiment valid if it never is reproduced? Perhaps the reproducibility is what makes co-discoveries from different labs so exciting—the realization that a phenomenon is “real” and others notice it as well. This suggests that there is merit to multiple groups tackling similar questions. These issues are of considerable interest as the science community has faced concerns about the reproducibility of peer-reviewed data and, in extensively corrected or retracted papers, if the remaining data has value in a given manuscript. Footnote 21
The early outcomes of our experimental reworking were both frustrating and dissatisfying. We had success in observing localized necrotic lesions and abscission, but wondered if abscission was the gold-standard bioassay to score peppers for TMV resistance. Did contemporaries of Holmes confirm his findings or report useful modifications that we were unaware of when we initiated our experimental reworking? Is this method used today to evaluate TMV resistance in Capsicum ?
This sort of closer reading and (re-) interpretation is integral to science practice. For example, Staffan Müller-Wille and Giuditta Parolini inspected copies of Mendel’s pea breeding manuscript, finding that readers “actively engaged with the text” by “rehearsing calculations and by employing Mendel’s notation system” (Müller-Wille & Parolini, 2020 , p. 157). The annotations and underlining revealed what the reader “deemed most important” (Müller-Wille & Parolini, 2020 , p. 153). Today, genetics students learn to use Punnett’s square for visualizing the outcome of genetic crosses of dominant and recessive genes. This “interplay between text and image” (Müller-Wille & Parolini, 2020 , p. 163) and annotation, for us, was an important part of a process that revealed Holmes’ ideas, to identify experimental materials and methods, and to design complementary experiments. Later, we repeated this process as a tool to troubleshoot possible errors in reworking the pepper experiment. As Müller-Wille and Parolini write, this “active engagement” is a fundamental aspect of research “alongside the observations conducted at the lab bench and the experimental garden” to interpret and gain “practical knowledge” (Müller-Wille & Parolini, 2020 , pp. 164, 165). Similarly, Pamela Smith showed us the importance engaging with material objects, text and drawings, to recreate skills and knowledge of the past (Smith, 2012 ). Nils-Otto Ahnfelt and Hjalmar Fors also reported that they “should have returned to the sources and read the original recipes more carefully”; consulted other sources to inform a practice or provide an “indirect pointer” for troubleshooting and problem-solving; and, performed “complementary” experiments with modern instrumentation to replicate the historical work (Ahnfelt & Fors, 2016 , p. 177).
To evaluate the reproducibility of Holmes’ experiment in others’ hands, across a gap of nine decades, we identified 17 manuscripts, from 1936 to 2021, that reported on TMV inoculation of L -gene pepper plants and also cited Holmes’, 1934 or 1937 Phytopathology papers (Table 3 ). Of these papers, all 17 reported LNLs following inoculation of L -gene pepper plants with TMV; 12 also reported leaf abscission. Two authors reported they obtained L -gene bell pepper seed from Holmes (Greenleaf, 1953 ; Murakishi, 1960 ). Harry Murakishi, at Michigan State University, used Holmes’ “LL-resistant garden pepper”, reporting LNLs appeared following TMV inoculation, but he did not indicate leaf abscission (Murakishi, 1960 , p. 464). Walter H. Greenleaf, a pepper breeder at Auburn University, regularly exchanged seed and viruses with Holmes, and on several occasions reported that Holmes’ LL -bell pepper responded to TMV inoculation with LNLs and abscission (Greenleaf, 1953 , 1986a , 1986b ). Greenleaf also used Holmes’ L -gene bell pepper line to develop a TMV-resistant pimiento pepper (Greenleaf et al., 1969 ).
H. H. McKinney, a USDA plant virologist, found that TMV-inoculated Capsicum frutescens ( L -gene) plants maintained at 23ºC developed “local necrotic lesions on mature and nearly mature leaves … and these leaves eventually absciss” (McKinney, 1937 , p. 55). Similarly, Glenn S. Pound and G. P. Singh at the University of Wisconsin, noted “necrotic lesions on inoculated leaves. At all temperatures, inoculated leaves abscised and the plants remained free of systemic infection” (Pound & Singh, 1960 , p. 805). In 1968, Mo-Yeong Lee and Paul G. Smith at the University of California-Davis, scored pepper lines for TMV-resistance by the LNLs response “just before leaf abscission” (Lee & Smith, 1968 , p. 1445). But twelve papers, most of which did not cite Holmes' TMV-pepper work, did not mention abscised leaves in response to TMV infection, suggesting that it was not a consistently reliable assay (as we found) or it added no additional value to the standard scoring for LNLs.
Donna Bilak and her colleagues in the Making and Knowing Project, remind us that “recipe literature is a challenging genre to read, not only because of its frequent technical obscurity and abridged prose, but often even more so because of its simple style and apparent straightforwardness” (Bilak et al., 2016 , p. 41). The same can be said the materials and methods sections of peer-reviewed scientific manuscripts and protocol manuals. Footnote 22 Ken Albala, a culinary historian and practitioner, reminds us of the importance of Renaissance cooks who “recorded their extensive experience” even if the methods are unfamiliar to us; in short, “we must trust what is on the page” (Albala, 2010 , p. 87). Similarly, the experimental work of Lawrence Principe reveals that reconstruction of the alchemy is possible because the work is “grounded in chemical reality, even though a simple reading of the text by a person well-versed in chemistry might well suggest the contrary” (Principe, 1987 , p. 27). Yet, peer-reviewed manuscripts often have insufficient details to reproduce experiment. And becoming adept with new techniques and tools, such as construction of a TEA laser (Collins, 1974 ), may require communication with the innovators, spending many frustrating weeks to years to become expert in the method, or waiting for a commercial company to develop a kit, machine, or service to standardize the technique.
In our pursuit of a decades old experiment using established, standard methodology we found nearly every element (soil, watering regimen, plants, lighting, inoculum, and pest control measures) affected our ability to make and learn from Holmes. This has ramifications for scientists and historians who decide to replicate key experiments in their field. As we show here, and as discussed by Jordan and Lynch for plasmid preps, success with a particular protocol belies the depth of required experience and expertise by the users. Our difficulty in recapitulating Holmes’ work was difficult and interesting, and we learned a few things:
1. We became better readers and observers. As noted by Pamela Smith and others, we too stumbled on the processual research, providing us with the opportunity (and necessity) of studying processes rather than discrete events, to carefully read the materials and methods, and then assemble the needed reagents and tools. This extends into the need for carefully reading the protocols following failed attempts. In the early stages, the process of re-investigation seemed straightforward. Yet it quickly became evident that we were missing or unable to identify the materials used by Holmes. We interpreted what we expected to observe; that is, we anticipated ‘seeing’ the exact outcomes shown in Fig. 1 , but we had not realized that a particular combination of plant age, lighting and temperature would determine the outcome. We did not review the literature citing Holmes, as we were intent on reworking his experiment. However, this literature revealed that LNLs were sufficient to identify TMV-resistance plants. Leaf abscission offered no added value when studying L -gene pepper plants or developing new commercial varieties.
2. Side projects are projects. The TMV-pepper experiment was piggy-backed onto ‘normal’ experiments in our molecular virology laboratory. We do not often perform experiments unrelated to our primary research interests. “What is typical, rather, are extended series of experiments which communicate among each other with different intensity and constitute an experimental texture,” as noted by Hans-Jorg Rheinberger (Rheinberger, 2001 , p. 53). The TMV experiment dislocated us from our normal science practice, re-enforcing that that tacit knowledge and its implicit peculiarities are relevant to the success of the practitioner (Keller, 1984 ). We greatly underestimated the time and effort required to rework this experiment, because we were confident that the experiments were simple, and could be managed as a ‘side project’. Instead, we found that it takes time, money, and intensive focus to rework an experiment—there are no shortcuts.
Repeating this ‘simple’ experiment cast doubt on our expertise, leading us to revert to our familiarity with recombinant DNA tools to visualize the results. In Jordan and Lynch’s study of the plasmid prep, interviews with practitioners made evident that there is both a “black box” aspect and a “reflective” aspect to this work, and an individual in a laboratory (and an individual laboratory), may have strong feelings “over just what sorts of variations are tolerable, trivial, or significant” (Jordan & Lynch, 1992 , p. 105). It is inevitable that we work with available materials and these may change over time (plant lines, virus strains), specific conditions (laboratory infrastructure) and expertise (Holmes’ experiences versus our experiences). From the outlines and framework presented by Holmes, we have re-realized the complexity of our ‘everyday’ work in the laboratory. The devil is in the details.
3. Reproducibility is experimentation. We were humbled by the complexity of repeating an experiment from 1934. What we found, reiterating the analysis by other re-workers in the making and knowing community, is that written materials and methods are important, but are not technical guides or how-to manuals to replicate an experiment. The repetition, frustration, and mastery of techniques are part and parcel of doing science. Of equal importance are the ineffable influences of mentors and peers, institutions, classroom knowledge and laboratory training and a dash of serendipity that affect the successes, failures, interpretation and presentation of data.
The difficulty of repeating this work speaks to a larger issue in the biological science—reproducibility. Reproducibility in science has relevance to scientists, historians, philosophers, publishers and funding agencies. Journals provide guidelines to authors, emphasizing that the materials and methods should be sufficiently detailed for the work to repeated or replicated. Footnote 23 Yet, from our ‘simple’ experimental reworking, the written manuscript was not sufficient—crucial information was found in a photograph (Fig. 1 ). Perhaps this not surprising, because images (drawings, photographs, video, and models) “are especially effective in organizing technical knowledge into an abbreviated form” (Smith, 2012 , p. 24).
Pamela Smith and Hasok Chang have shown us through their historiographic reconstructions that the text and even drawings are not enough; making is process of “observation and imitation” of experts, oftentimes requiring complementary experiments (Chang, 2011 ; Smith, 2012 , p. 10). The repetitive nature of doing science, familiar to any laboratory researcher, is normal science (Jordan & Lynch, 1992 ). This “repeated trial and error was ‘skill’” acquired by attention and focus, such as hands-on laboratory experiences (Smith, 2012 , p. 26). Historical reconstructions, whether of artisan crafts, counting seeds, measuring the boiling point of water, or TMV-inoculation, can be used to address what is perceived to be a reproducibility “crisis” in science. In every instance, historiographic reconstruction has shown the impracticability of exactness in reproducing written work. For us, as scientists and practitioner-historians, we have been excited by how closely our reworking reflects ‘normal science’ by using journal articles, protocol manuals, and in-house experience to plan, perform and evaluate an experiment. Along the way modifications and changes occur, sometimes becoming normalized practice in a laboratory. Scientific manuscripts outline how work was performed, they are narratives of new findings, on the path towards new, even significant, advances in a field of study. If the work is subjected to replication then, by the very nature of scientific practice, somewhat different outcomes may be reported. Hasok Chang, using the term “extension” pushes this point, as we interpret it: Does the historian become a scientist, or considered to be practicing science when the reworking or complementary experiments lead to “something new (though old) about nature” or “genuine original contributions to scientific knowledge”? (Chang, 2011 , p. 324). And, vice versa: are scientists who reproduce recently published findings from another laboratory acting as historians of science?
Another aspect of reproducibility is choice. Replicative experiments do not have the scientific prestige of original work, despite the fact that the financial outlay (salaries, reagents, equipment, and publication costs) is equivalent to discovery-based research. Which experiments will be tested for reproducibility? For a specific example, which of 200,000 COVID-19 manuscripts published the past 18 months should be replicated? Footnote 24 As shown by Johan Chu and James Evans, most “scholarly attention” is focused on highly cited papers from well-known labs, making it difficult for “less-established papers—even those with novel, useful, and potentially transformative ideas” to gain attention (Chu & Evans, 2021 ).
Of papers subjected to replication, how one approaches an experiment is predicated on many parameters, including biased approaches and interpretations. Footnote 25 The influence(r)s guiding the reworking of a particular experiment; interpreting and troubleshooting results; and which findings should be emphasized will differ—even for scientists working together. For example, our reworking of Holmes’, 1934 experiment could be judged unsuccessful: our plants did not look exactly the same, we did not have identical results, and we relied on complementary methods. But, upon reflection, our self-analyses was too harsh. In fact, we learned about the complexity of reworking by localizing temporal and material constraints, identifying decades of changes to “normal” science (training, tools and regents) and making use of advances in virology to understand Holmes’ findings. Importantly, peer-reviewed manuscripts that cited Holmes work, showed us that the LNLs assay, not abscission, remained the standard by which plants were (and are) scored for resistance to TMV.
6 Local knowledge and placelessness
In their exploration of allosteric regulation, Angela Creager and Jean-Paul Gaudillière focused on the role of local knowledge and the co-evolution of meaning and experiments within individual locations (Creager & Gaudillière, 1996 , p. 90). We interpreted Holmes’ meaning and intent as we reworked his experiment. Robert Kohler has stated that laboratories “are simplified and standardized, stripped of all context and environmental variations; they are places apart from the world—placeless places. It is this odd spatial quality that gives knowledge produced in labs its credibility. The simplicity and sameness of labs helps ensure that experiments turn out the same wherever they are done, which is one of the main reasons why we trust experiment more than other ways of knowing” (Kohler, 2002 , p. 191). Yet we did not experience this, and such difficulties in repeating what may be considered normalized science call into question Robert Kohler’s idea of a laboratory as a “placeless place”. As with Creager and Gaudillière’s historiography of allosteric regulation experiments in Berkeley and Paris, we (and Holmes) “worked with different systems, local habits, and distinctive strategies for making decisions” (Creager & Gaudillière, 1996 , p. 3). Although we “envisioned” we were “working on the same problems and being part of the same group,” in our case studying TMV, the “decisions made and observations found in each setting affected choices and possibilities” (Creager & Gaudillière, 1996 , p. 3). Thus, we had to temper our expectations as we worked to replicate Holmes’ experiments.
In our instance, we were separated not by an ocean, but by time. To perform our experiments we made assumptions about Holmes’ experiments across a gap of decades, yet we “envisioned” ourselves as working on the same problem. Our experiments were informed by Holmes, reconstructing as best we could, his materials and methods. We obtained similar, but not identical results. We learned that location, practice, and a “feeling” for the tools/objects/agents matter greatly when re-working and re-assessing any experiment of the past (Chang, 2011 ; Creager & Gaudillière, 1996 ; Keller, 1984 ; Kohler, 2002 ).
Again, from Smith, we were reminded that “experiential” or makers’ knowledge is gained by the experimental habit of “doing things over and over” and she (and we) “marveled at the length of time it took to acquire experiential knowledge”(Smith, 2012 , pp. 22–23). We found that reworking Holmes’ experiment differed little from initiating a new project including the attendant pitfalls, problem-solving, and interpretation of the results—we had to become wholly immersed in the process of practice. That Holmes intuited the presence of a host resistance gene to TMV infection from an observation of localized necrotic lesion and leaf abscission, shows us a scientist who mastered the craft of working with his research tools, to make foundational advances in virology.
For recent scholarship on the history of tobacco mosaic virus and F. O. Holmes’ contributions to advances in plant virology, see Creager ( 2002 ), Creager & Morgan ( 2008 ), Scholthof ( 2004 , 2014 , 2016 ), Scholthof & Peterson ( 2006 ), Scholthof et al. (1999).
The importance of the efforts of the US government and scientists to collect and distribute seed to plant breeders and farmers in the United States has been addressed by Campbell et al., (1999), Curry ( 2016 , 2019 ), Fitzgerald ( 1990 ), Fullilove ( 2017 ), Kingsbury ( 2011 ), Kloppenburg Jr. (2005), Palladino ( 1991 ).
For all the successes attributed to plant breeding and genetic resistance to pathogens, a history has yet to be written of the formative years of scientific breeding for plant disease resistance. Textbooks on plant genetics and plant breeding and journals such as Phytopathology , Crop Science , Agronomy and Genetics are helpful in understanding the state-of-the art in the early twentieth century to the present. “Plant Pathology: Problems and Progress, 1908–1958” provides an overview of advances in plant pathology (Holton et al., 1959 ); in this volume, Holmes wrote a commentary on plant virology (Holmes, 1959 ).
On the science and technology of plant breeding and plant genetics in the United States in the early to mid-twentieth century, see Curry ( 2016 ), Fitzgerald ( 1990 ), Fullilove ( 2017 ), Kingsbury ( 2011 ).
We can speculate that this almost certainly is due to ploidy; that is, the genus Capsicum is diploid allowing for straightforward interpretation of species crosses by Mendelian genetics. In contrast, many of the species in Nicotiana are polyploid which confounds interpretation of genetic outcomes and introgression of genes of interest. We have addressed the difficulties Holmes had towards introgression of the resistance gene N to commercial tobacco from N. glutinosa (Scholthof, 2016 ).
By 1959, the USDA Farmers’ Bulletin referred growers to Rutgers World Beater No. 13, Yolo Wonder, Keystone Resistant Giant, and Liberty pepper lines, reporting “considerable resistance” to TMV (Boswell et al., 1959 , p. 27).
In “Doing Biology,” Joel Hagen, Douglas Allchin and Fred Singer used case studies or “historical episodes” of work by individual scientists “that exemplify important characteristics of scientific practice” to “more fully reveal how biology is done” within the context of “science-in-the-making” (Hagen et al., 1997, pp. vi, vii). This book is not a hands-on guide, instead it shows the complexity of science, decision making, and interpreting outcomes with the broad area of the history and sociology of science. Furthermore, common practice(s) also change. What were once standard techniques for plant pathologists are no longer learned in graduate (practicum) courses or the research laboratory. Instead, “hands on” experiences increasingly are replaced with lectures and journal clubs.
“TEA” refers to the Transversely Excited Atmospheric Pressure CO 2 laser, or TEA laser. In Collins’ paper the TEA set refers to the interactions between laboratories in North America and the U.K. who are have developed or are keen to develop the apparatus. Here, Collins discusses networks and networking between labs and how ideas and knowledge travel (Collins, 1974 ).
These recruits “included two plant pathologists, two professors of agronomy, one professor of philosophy (originally trained as a biologist), four biologists, one [human] computer, one practical corn breeder, and one professor and three assistants in plant physiology.” Two of these men by “birth, early life and education” belonged to “the ‘corn belt’ section of the country, and are thoroughly and intimately familiar with maize” and “had experience in corn judging” (Pearl, 1930 , pp. 126, 127). We relay this to emphasize the difficulty of reworking experiments; learning the craft behind the experimental technique is key to making and knowing (Smith, 2012 ).
It is important to note that this work on (scientific) material culture is constrained by university rules, and federal and state laws. Laboratory work with viruses and plant materials requires a permit and all lab members be trained in the use of biological materials, chemicals, and (recombinant) pathogens. In this instance, biological materials were used with the approval of the Texas A&M University Institutional Biosafety Committee (Permits IBC2016-130 and IBC2019-139).
As reported by Holmes ( 1934 ), TMV was maintained on tobacco ( N. tabacum ), as inoculum for the Capsicum experiments.
The seeds were sown in well-moistened Sungro brand potting mix and transferred to a growth chamber set at ~ 25 °C, 16 h light, 8 h dark ~ 22 °C, 60% humidity, 100–120 μmol/m 2 /s light intensity, watered 3 times/week and fertilized with 20–20-20 (N-P-K) once a week (20 ml/L). Following inoculation, the plants were placed in the laboratory on a plant growth rack at ~ 23 °C 16 h light, 8 h night, and watered 3 times/week without the addition of fertilizer.
Of importance to our study, Eric Votava and Paul Bosland reported “‘California Wonder’ should not be included as a standard control in other Capsicum research. … The concept of a standard control entails that the ‘control’ can be used in separate labs and in separate experiments and act as a consistent and repeatable benchmark. The dependability of a sample to act as a standard control is cast into doubt if it is shown to contain a high degree of variability or if even a high degree of variability is suspected” (Votava & Bosland, 2002 , p. 1102). In 1937, Holmes reported that not all plants of a seed lot responded similarity to infection, suggesting the possibility of a “contaminant of the seed lot” due to inadvertent mixing of seeds (Holmes, 1937 , p. 641). Rachel Ankeny and Sabina Leonelli provide an HPS-centered discourse on standard or wildtype organisms (Ankeny & Leonelli, 2021 ).
For example, in the USDA National Germplasm Collection, ~ 15 lines of Tabasco are identified within the Capsicum frutescens accessions ( https://npgsweb.ars-grin.gov/gringlobal/search ). Commercial seed companies then advance lines with specific traits (yield, maturity, fruit color, etc.).
The mock-inoculated leaves (control) and TMV-inoculated leaves of Tabasco and bell pepper were photographed several timepoints (days post-inoculation; dpi). Each independent experiment used three susceptible and three resistant plants and the experiments were repeated several times.
The infectious cDNA used for these experiments is based on the U1 (common or type) strain (Siegel & Wildman, 1954 ). This strain, as reported by Milton Zaitlin and H. W. Israel, from “personal recollections of C. A. Knight, W. C. Price and F. O. Holmes suggest that the original isolate used by W. M. Stanley came from J. Johnson of the Univ. of Wisconsin via L. O. Kunkel. The U1 strain (Wittmann & Wittmann-Liebold, 1963 ) and the German strain ‘vulgare’ (Wittmann-Liebold & Wittmann, 1967 ) also came from Johnson” (Zaitlin & Israel, 1975 ).
Specifically, we used a binary plasmid containing the coding sequence of TMV with a gfp insert (pJL24) (Lindbo, 2007 ). This plasmid was propagated in Agrobacterium tumefaciens strain GV3101, then infiltrated into N. benthamiana or N. tabacum cv. Turk leaves to initiate the transcription of the recombinant viral genome, TMV-GFP.
The California Wonder variety, lacking resistance to TMV, was released in 1937, having first been selected by a California grower in 1928 (Boswell, 1937 ; Votava & Bosland, 2002 ). Paul W. Bosland, the New Mexico State University pepper breeder, has documented an exhaustive list of garden catalog descriptions of pepper varieties and the year of commercial release. Bell pepper varieties were bred for TMV resistance by genetic introgression of the Tabasco L -gene. Two examples were California Wonder 300 (XP 300) a “thick walled, blocky California Wonder type” released by Asgrow Seed in 1966; and, California Wonder 300 TMR, released in 1999 by Carolina Seeds with “glossy green, thick walled fruit, which turn green to red at maturity, smooth blocky fruit, mostly 4-lobed, averaging 110 × 100 mm fruit size, 72 days to harvest” (Bosland, 2019 ). https://cucurbitbreeding.wordpress.ncsu.edu/2016/06/03/pepper-a-l/ .
The 2019 Burpee’s catalog description of Heirloom California Wonder did not indicate TMV resistance.
The rapid acceptance of Holmes’ local lesion method and the use of N. glutinosa for this bioassay by plant virologists was discussed previously (Scholthof, 2011 , 2014 ).
This “reproducibility crisis” is discussed by Marcia McNutt, president of the National Academies of Science and former editor-in-chief of Science (McNutt 2014 ); Jeremy Berg, also a former editor-in-chief of Science (Berg 2019 ); and, a Nature collection on “challenges in irreproducible research” at https://www.nature.com/collections/prbfkwmwvz .
Angela Creager provides an interesting discussion of the use of laboratory manuals by practitioners (Creager, 2020 ). This and other articles in the BJHS Themes issue “Learning by the Book: Manuals and Handbooks in the History of Science” edited by Elaine Leong, Angela Creager, and Mathias Grote is a particularly helpful volume to grasp the importance of text and its interpretation to perform and interpret science. Peer-reviewed manuscripts should, but mostly do not, include sufficiently detailed materials and methods to allow the work to be reproduced. How-to videos offer an alternative or supplemental format to communicate the use of materials and methods; an example is JoVE , a peer-reviewed journal of visual experimentation (jove.com).
Similarly, the National Institutes of Health (NIH) has policy and compliance guidelines for “Rigor and Reproducibility” for all grant proposals ( https://grants.nih.gov/policy/reproducibility/index.htm ). The guidelines, fully implemented in 2020, assert that the “application of rigor ensures robust and unbiased experimental design, methodology, analysis, interpretation, and reporting of results. When a result can reproduced by multiple scientists, it validates the original results and readiness to progress to the next phase of research” ( https://www.nih.gov/research-training/rigor-reproducibility ). In an extension of this, authors of a recent feature article in eLife , a highly ranked journal in the life sciences, suggested the establishment of communities of “'rigor champions' who are committed to promoting rigor and transparency in research” (Koroshetz et al., 2020 ).
Searching “COVID” at PubMed ( https://pubmed.ncbi.nlm.nih.gov/?term=covid ) yielded 194,281 publications on 15 November 2021. The Web of Science Core Collection ( https://clarivate.com ) identified 193,280 publications of which ca. 7,764 (4%) are highly cited (accessed 15 November 2021). “Retraction Watch” has recorded 197 retracted or problematic papers ( https://retractionwatch.com/retracted-coronavirus-covid-19-papers/ ; accessed 15 November 2021).
A recent report by the National Academies of Science, Engineering and Medicine (2019) addressed these topics in Reproducibility and Replicability in Science , with a particular focus on replication in chapter 7.
Agrios, G. N. (1978). Plant pathology . Elsevier Academic Press. Doi: https://doi.org/10.1016/B978-0-12-044560-8.50018-1
Ahnfelt, N.-O., & Fors, H. (2016). Making early modern medicine: Reproducing Swedish bitters. Ambix, 63 , 162–183. https://doi.org/10.1080/00026980.2016.1212886
Article Google Scholar
Ahnfelt, N.-O., Fors, H., & Wendin, K. (2020). Historical continuity or different sensory worlds? What we can learn about the sensory characteristics of early modern pharmaceuticals by taking them to a trained sensory panel. Berichte zur Wissenschaftsgeschichte, 43 , 412–429. https://doi.org/10.1002/bewi.202000009
Albala, K. (2010). Cooking as research methodology: Experiments in Renaissance cuisine. In J. Fitzpatrick (Ed.), Renaissance food from Rabelais to Shakespeare: Culinary readings and culinary histories (pp. 73–88). Ashgate.
Google Scholar
Ankeny, R., & Leonelli, S. (2021). Model organisms . Cambridge University Press.
Barwich, A.-S., & Rodriguez, M. (2020). Fashion fades, Chanel No. 5 remains: Epistemology between style and technology. Berichte zur Wissenschaftsgeschichte, 43 , 367–384. https://doi.org/10.1002/bewi.202000006
Berg, J. (2019). Replication challenges. Science, 365 , 957. https://doi.org/10.1126/science.aaz2701
Bilak, D. (2020). Out of the Ivy and into the Arctic: Imitation coral reconstruction in cross-cultural contexts [ https://doi.org/10.1002/bewi.202000010 ]. Berichte zur Wissenschaftsgeschichte , 43 , 341–366. https://doi.org/10.1002/bewi.202000010
Bilak, D., Boulboullé, J., Klein, J., & Smith, P. H. (2016). The making and knowing project: Reflections, methods, and new directions. West 86th: A Journal of Decorative Arts Design History, and Material Culture, 23 , 35–55. https://doi.org/10.1086/688199
Bosland, P. W. (2019). Vegetable cultivar descriptions for North America – Pepper (A-L) . https://cucurbitbreeding.wordpress.ncsu.edu/2016/06/03/pepper-a-l/
Boswell, V. R. (1937). Yearbook of agriculture: Improvement and genetics of tomatoes, peppers, and eggplant . U.S. GPO
Boswell, V. R., Doolittle, S. P., Pultz, L. M., Taylor, A. L., & Campbell, R. E. (1959). Pepper production, disease and insect control . US GPO http://hdl.handle.net/2027/chi.59177489
Campbell, C. L., Peterson, P. D., & Griffith, C. S. (1999). The formative years of plant pathology in the United States . APS Press.
Chang, H. (2011). How historical experiments can improve scientific knowledge and science education: The cases of boiling water and electrochemistry. Science and Education, 20 , 317–341. https://doi.org/10.1007/s11191-010-9301-8
Chu, J. S. G., & Evans, J. A. (2021). Slowed canonical progress in large fields of science. Proceedings of the National Academy of Sciences, 118 , e2021636118. https://doi.org/10.1073/pnas.2021636118
Collins, H. M. (1974). The TEA set: Tacit knowledge and scientific networks. Science Studies, 4 , 165–185. https://doi.org/10.1177/030631277400400203
Creager, A. N. H. (2002). The life of a virus: Tobacco mosaic virus as an experimental model, 1930–1965 . University of Chicago Press.
Creager, A. N. H. (2020). Recipes for recombining DNA: A history of molecular cloning: A laboratory manual . BJHS Themes, 5 , 225–243. https://doi.org/10.1017/bjt.2020.5
Creager, A. N. H., & Gaudillière, J.-P. (1996). Meanings in search of experiments and vice-versa: The invention of allosteric regulation in Paris and Berkeley, 1959–1968. Historical Studies in the Physical and Biological Sciences, 27 , 1–89. https://doi.org/10.2307/27757769
Creager, A. N. H., & Morgan, G. J. (2008). After the double helix: Rosalind Franklin’s research on Tobacco mosaic virus. Isis, 99 , 239–272. https://doi.org/10.1086/588626
Curry, H. A. (2016). Evolution made to order: Plant breeding and technological innovation in twentieth-century America . University of Chicago Press.
Curry, H. A. (2019). Why save a seed? Isis, 110 , 337–340. https://doi.org/10.1086/703337
Dijkstra, J., & De Jager, C. P. (1998). Protocol 1: Mechanical inoculation of plants. In Practical plant virology: Protocols and exercises (pp. 5–13). Springer-Verlag. https://link.springer.com/content/pdf/10.1007%2F978-3-642-72030-7.pdf
Fitzgerald, D. K. (1990). The business of breeding hybrid corn in Illinois, 1890–1940 . Cornell University Press.
Ford, R., & Evans, T. (2003). Tobacco mosaic virus. Plant Health Instructor . https://doi.org/10.1094/PHI-K-2003-0528-01
Fors, H., Principe, L. M., & Sibum, H. O. (2016). From the library to the laboratory and back again: Experiment as a tool for historians of science. Ambix, 63 , 85–97. https://doi.org/10.1080/00026980.2016.1213009
Fullilove, C. (2017). The profit of the Earth: The global seeds of American agriculture . University of Chicago Press.
Greenleaf, W. H. (1953). Effects of tobacco-etch virus on peppers ( Capsicum sp.). Phytopathology, 43 , 564–570.
Greenleaf, W. H. (1986a). Pepper breeding. In M. J. Bassett (Ed.), Breeding vegetable crops (pp. 67–134). AVI Pub. Co.
Greenleaf, W. H. (1986b). The tabasco story. HortScience, 10 , 98.
Greenleaf, W. H., Hollingsworth, M. H., Harris, H., & Rymal, K. S. (1969). Bighart: Improved variety of pimiento pepper. Auburn University Agricultural Experiment Station, Leaflet No., 78 , 1–8.
Hagen, J. B., Allchin, D., & Singer, F. (1997). Doing biology . Benjamin Cummings. doingbiology.net
Hendriksen, M. M. A. (2020). Rethinking performative methods in the history of science. Berichte zur Wissenschaftsgeschichte, 43 , 313–322. https://doi.org/10.1002/bewi.202000017
Hendriksen, M. M. A., & Verwaal, R. E. (2020). Boerhaave’s furnace. Exploring early modern chemistry through working modells. Berichte zur Wissenschaftsgeschichte, 43 , 385–411. https://doi.org/10.1002/bewi.202000005
Holmes, F. O. (1928). Accuracy in quantitative work with tobacco mosaic virus. Botanical Gazette, 86 , 66–81. https://doi.org/10.1086/333873
Holmes, F. O. (1929a). Inoculating methods in tobacco mosaic studies. Botanical Gazette, 87 , 56–63. https://doi.org/10.1086/333924
Holmes, F. O. (1929b). Local lesions in tobacco mosaic. Botanical Gazette , 87 , 39–55. https://doi.org/10.1086/333923
Holmes, F. O. (1931). Local lesions of mosaic. Nicotiana Tabacum L. Contributions of the Boyce Thompson Institute 3 , 163–172.
Holmes, F. O. (1932). Symptoms of tobacco mosaic disease. Contributions of the Boyce Thompson Institute, 4 , 323–357.
Holmes, F. O. (1934). Inheritance of ability to localize tobacco-mosaic virus. Phytopathology, 24 , 984–1002.
Holmes, F. O. (1937). Inheritance of resistance to tobacco-mosaic disease in the pepper. Phytopathology, 27 , 637–642.
Holmes, F. O. (1938). Inheritance of resistance to tobacco-mosaic disease in tobacco. Phytopathology, 28 , 553–561.
Holmes, F. O. (1959). Discussion of chapters XLV and XLVI. In C. S. Holton, G. W. Fischer, R. W. Fulton, H. Hart, & S. E. A. McCallan (Eds.), Plant pathology: Problems and progress 1908–1958 (pp. 521–523). University of Wisconsin Press.
Holton, C. S., Fischer, G. W., Fulton, R. W., Hart, H., & McCallan, S. E. A. (Eds.). (1959). Plant pathology: Problems and progress, 1908–1958 . Univerisity of Wisconsin Press.
Jordan, K., & Lynch, M. (1992). The sociology of a genetic engineering technique: Ritual and rationality in the performance of the “plasmid prep.” In A. E. Clarke & J. H. Fujimura (Eds.), The right tools for the job: At work in twentieth-century life sciences (pp. 77–114). Princeton University Press.
Chapter Google Scholar
Kalmus, H., & Kassanis, B. (1945). The use of abrasives in the transmission of plant viruses. Annals of Applied Biology, 32 , 230–234. https://doi.org/10.1016/S0065-3527(08)60527-8
Keller, E. F. (1984). A feeling for the organism: The life and work of Barbara McClintock . Henry Holt.
Kingsbury, N. (2011). Hybrid: The history and science of plant breeding . University of Chicago Press.
Kloppenburg Jr., J. R. (2005). First the seed: The political economy of plant biotechnology (2nd ed.). University of Wisconsin Press.
Kohler, R. E. (2002). Place and practice in field biology. History of Science, 40 , 189–210. https://doi.org/10.1177/007327530204000204
Koroshetz, W. J., Behrman, S., Brame, C. J., Branchaw, J. L., Brown, E. N., Clark, E. A., Dockterman, D., Elm, J. J., Gay, P. L., Green, K. M., Hsi, S., Kaplitt, M. G., Kolber, B. J., Kolodkin, A. L., Lipscombe, D., MacLeod, M. R., McKinney, C. C., Munafò, M. R., Oakley, B., . . . Silberberg, S. D. (2020). Framework for advancing rigorous research. eLife , 9 , e55915. Doi: https://doi.org/10.7554/eLife.55915
Lee, M.-Y., & Smith, P. G. (1968). Identification of the L gene for tobacco mosaic resistance in three pepper species. Phytopathology, 68 , 1445.
Lindbo, J. A. (2007). High-efficiency protein expression in plants from agroinfection-compatible tobacco mosaic virus expression vectors. BMC Biotechnology , 7 , 52.
McKinney, H. H. (1937). Virus mutation and the gene concept. Journal of Heredity, 28 , 51–57.
McNutt, M. (2014). Reproducibility. Science, 343 (6168), 229. https://doi.org/10.1126/science.1250475
Müller-Wille, S., & Parolini, G. (2020). Punnett squares and hybrid crosses: How Mendelians learned their trade by the book. BJHS Themes, 5 , 149–165. https://doi.org/10.1017/bjt.2020.12
Murakishi, H. H. (1960). A necrotic pod streak of pepper caused by tobacco mosaic virus. Phytopathology, 50 , 464–466.
National Academies of Sciences, Engineeering and Medicine. (2019). Reproducibility and replicability in science . The National Academies Press. https://doi.org/10.17226/25303
Palladino, P. (1991). The professionalism of plant breeding in the United States: The technology of hybridisation and its consequences. Minerva, 29 , 507–515. https://doi.org/10.1007/BF01113493
Pearl, R. (1911). The personal equation in breeding experiments involving certain character of maize. Biological Bulletin, 21 , 339–366. https://doi.org/10.2307/1536152
Pearl, R. (1930). Introduction to medical biometry and statistics (2nd ed.). W. B. Saunders . https://catalog.hathitrust.org/Record/001581750/Home
Pound, G. S., & Singh, G. P. (1960). The effect of air temperature on multiplication of tobacco mosaic virus in susceptible and resistant pepper. Phytopathology, 50 , 803–807.
Principe, L. (1987). “Chemical translation” and the role of impurities in alchemy: Examples from Basil Valentine's Triumph-Wagen . Ambix , 34 , 21–30.
Rheinberger, H.-J. (2001). History of science and the practices of experiment. History and Philosophy of the Life Sciences , 23 , 51–63.
Root-Bernstein, R. S. (1983). Mendel and methodology. History of Science, 21 , 275–295. https://doi.org/10.1177/007327538302100303
Scholthof, K.-B. G. (2004). Tobacco mosaic virus: A model system for plant biology. Annual Review of Phytopathology , 42 , 13–34. Doi: https://doi.org/10.1146/annurev.phyto.42.040803.140322
Scholthof, K.-B.G. (2011). TMV in 1930: Francis O. Holmes and the local lesion assay. Microbe, 6 , 221–225.
Scholthof, K.-B.G. (2014). Making a virus visible: Francis O. Holmes and a biological assay for Tobacco mosaic virus . Journal of the History of Biology, 47 , 107–145. https://doi.org/10.1007/s10739-013-9353-0
Scholthof, K.-B.G. (2016). Spicing up the N gene: F. O. Holmes and tobacco mosaic virus resistance in Capsicum and Nicotiana plants. Phytopathology, 107 , 148–157. https://doi.org/10.1094/PHYTO-07-16-0264-RVW
Scholthof, K.-B. G., & Peterson, P. D. (2006). The role of Helen Purdy Beale in the early development of plant serology and virology. Advances in Applied Microbiology , 59 , 221–241. Doi: https://doi.org/10.1016/S0065-2164(06)59008-2
Scholthof, K.-B. G., Shaw, J. G., & Zaitlin, M. (Eds.). (1999). Tobacco mosaic virus: One hundred years of contributions to virology . APS Press.
Sibum, H. O. (1995). Reworking the mechanical value of heat: Instruments of precision and gestures of accuracy in early Victorian England. Studies in History and Philosophy of Science Part A, 26 , 73–106. https://doi.org/10.1016/0039-3681(94)00036-9
Siegel, A., & Wildman, S. G. (1954). Some natural relationships among strains of tobacco mosaic virus. Phytopathology, 44 , 277–282.
Smith, P. H. (2012). In the workshop of history: Making, writing, and meaning. West 86th: A Journal of Decorative Arts . Design History, and Material Culture, 19 , 4–31. https://doi.org/10.1086/665680
Smith, P. H. (2016). Experiments in the early modern European investigation of nature. Viewpoint: The Magazine of the British Society for the History of Science , 110 , 8–9. http://www.bshs.org.uk/publications/viewpoint
Smith, P. H., & Beentjes, T. (2010). Nature and art, making and knowing: Reconstructing sixteenth-century life-casting techniques. Renaissance Quarterly, 63 , 128–179.
Usselman, M., Rocke, A., Reinhart, C., & Foulser, K. (2005). Restaging Liebig: A study in the replication of experiments. Annals of Science, 62 , 1–55. https://doi.org/10.1080/00033790410001711922
Votava, E. J., & Bosland, P. W. (2002). A cultivar by any other name: Genetic variability in heirloom bell pepper 'California Wonder'. HortScience 37 , 1100–1102. https://doi.org/10.21273/HORTSCI.37.7.1100
Wittmann, H. G., & Wittmann-Liebold, B. (1963). Tobacco mosaic virus mutants and the genetic coding problem. Cold Spring Harbor Symposia on Quantitative Biology, 28 , 589–595. https://doi.org/10.1101/SQB.1963.028.01.079
Wittmann-Liebold, B., & Wittmann, H. G. (1967). Coat proteins of strains of two RNA viruses: Comparison of their amino acid sequences. Molecular and General Genetics, 100 , 358–363. https://doi.org/10.1007/BF00334062
Zaitlin, M., & Israel, H. W. (1975). Tobacco mosaic virus (type strain). Description of Plant Viruses, Number 157 . https://www.dpvweb.net/dpv/showdpv/?dpvno=151
Download references
Acknowledgments
This work was funded in part by a National Science Foundation SES grant (No. 1456878) awarded to K.-B.G.S. W.B.C. was funded by an USDA-NIFA Predoctoral Award. We thank Angela Creager and Herman Scholthof for helpful, critical comments as we prepared this manuscript, as well the suggestions from the two anonymous referees.
Author information
Lorenzo J. Washington
Present address: Plant and Microbial Biology, University of California, Berkeley, CA, USA
April DeMell
Present address: Plant Biology, University of California, Davis, CA, USA
Maria R. Mendoza
Present address: FujiFilm Diosynth Biotechnologies, College Station, TX, USA
Will B. Cody
Present address: Chemical Engineering, Stanford University, Stanford, CA, USA
Authors and Affiliations
Plant Pathology and Microbiology, Texas A&M University, College Station, TX, 77843-2132, USA
Karen-Beth G. Scholthof
You can also search for this author in PubMed Google Scholar
Contributions
KBGS developed the project and wrote the manuscript; WBC, LJW, AD, and MRM performed the experiments; all authors contributed to the analysis of results, developing the figures, and manuscript edits.
Corresponding author
Correspondence to Karen-Beth G. Scholthof .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Scholthof, KB.G., Washington, L.J., DeMell, A. et al. Practicing virology: making and knowing a mid-twentieth century experiment with Tobacco mosaic virus . HPLS 44 , 3 (2022). https://doi.org/10.1007/s40656-021-00481-9
Download citation
Received : 24 May 2021
Accepted : 14 December 2021
Published : 01 February 2022
DOI : https://doi.org/10.1007/s40656-021-00481-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Science and experimentation
- Tobacco mosaic virus
- Plant genetics
- Plant pathology
- History of science
- Complementary science
- Reproducibility in science
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Tobacco mosaic virus has been known to cause a production loss for flue cured tobacco of up to two percent in North Carolina. [30] It is known to infect members of nine plant families, and at least 125 individual species, including tobacco, tomato , pepper (all members of the Solanaceae ), cucumbers , a number of ornamental flowers , [ 31 ] and ...
Tobacco Mosaic Virus is composed of 6% of RNA (not DNA) and protein. Frankel-Conrat and co-workers determined that the reconstitution of infective virus particles may occur after the association of protein subunits and RNA. Before this experiment, there were many experimental proofs that proved DNA being a genetic material.
Mar 24, 2020 · In 1929, biologist Francis Holmes used the tobacco mosaic virus to develop a method proving that viruses are discrete particles mixed in the filtered sap and that they have stronger effects at ...
The discovery saga of tobacco mosaic virus (TMV) begins with Adolf Mayer, Director of the Agricultural Experiment Station at Wageningen in The Netherlands. Mayer’s attention was first called to study the peculiar disease of tobacco in 1879. Although known since the middle of the 19th From the book Discoveries in Plant Biology, 1998, pp.: 105-110.
Mar 1, 1999 · Tobacco mosaic virus (TMV), as we now know the agent that Beijerinck and others were studying, was the first virus to be identified. Perhaps because of this, research on TMV and other plant viruses has continued to be of profound significance in addressing fundamental questions about the nature of viruses in general.
The history of tobacco mosaic virus (TMV) includes many firsts in science, beginning with its being the first virus identified. This review offers an overview of a history of research on TMV, with an emphasis on its close connections to the emergence and development of molecular biology.
Beijerinck's (1898) recognition that the cause of tobacco mosaic disease was a novel kind of pathogen became the breakthrough which eventually led to the establishment of virology as a science. Research on this agent, tobacco mosaic virus (TMV), has ...
Oct 5, 2016 · The P30 movement protein of tobacco mosaic virus is a single-strand nucleic acid binding protein. Cell. 1990 Feb 23; 60 (4):637–647. [Google Scholar] Citovsky V, McLean BG, Zupan JR, Zambryski P. Phosphorylation of tobacco mosaic virus cell-to-cell movement protein by a developmentally regulated plant cell wall-associated protein kinase.
classic reconstitution experiments, in which complete TMV was produced in vitro by mixing purified virus RNA and protein subunits (Fraenkel-Conrat and Williams, 1955), demonstrated that the information required for assembly is present in the structural components of the virus. Subsequent studies of the self-assembly of the virus have drawn
Feb 1, 2022 · Tobacco mosaic virus (TMV) has served as a model organism for pathbreaking work in plant pathology, virology, biochemistry and applied genetics for more than a century. We were intrigued by a photograph published in Phytopathology in 1934 showing that Tabasco pepper plants responded to TMV infection with localized necrotic lesions, followed by abscission of the inoculated leaves. This dramatic ...