

Literature Reviews: systematic searching at various levels
- for assignments
- for dissertations / theses
- Research Question Frameworks
- Search strategy and searching
- Boolean Operators
- Search strategy template
- Screening & critiquing
- Citation Searching
- Google Scholar (with Lean Library)
- Resources for literature reviews
- Adding a referencing style to EndNote
- Exporting from different databases
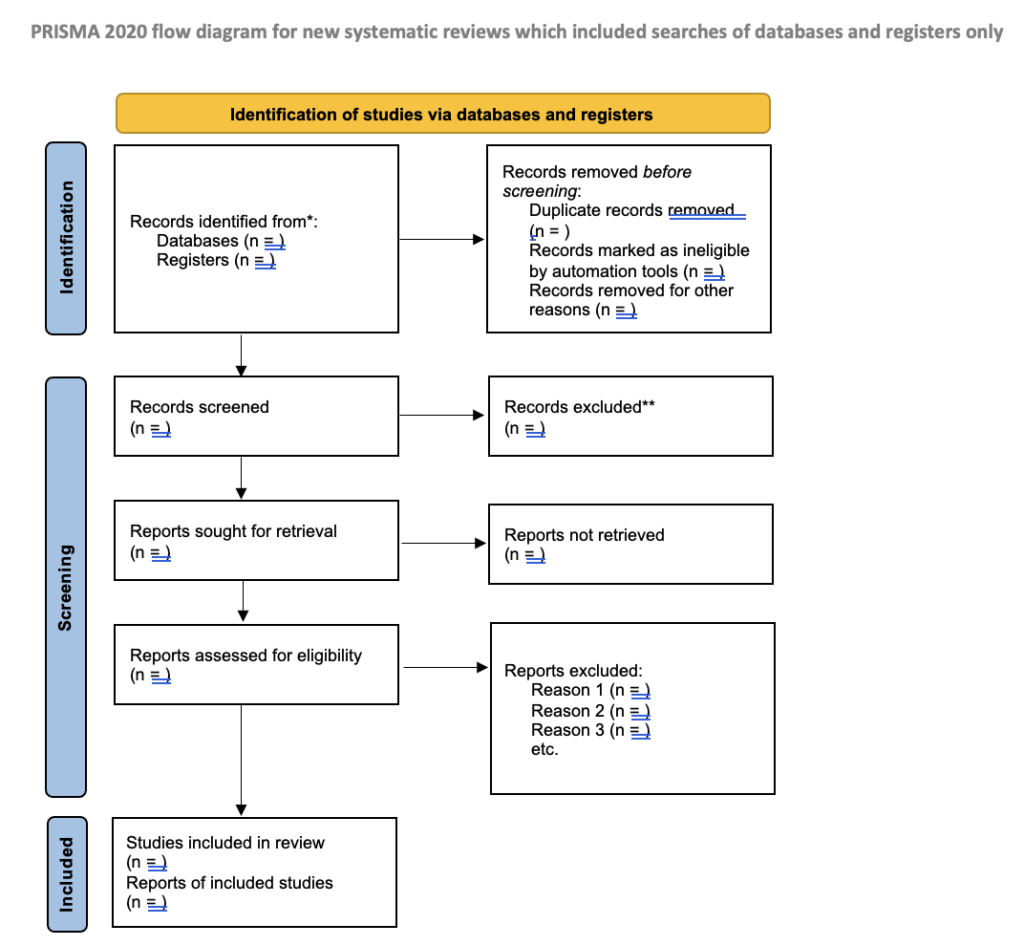
PRISMA Flow Diagram
- Grey Literature
- What is the PRISMA Flow Diagram?
- How should I use it?
- When should I use it?
- PRISMA Links
The PRISMA Flow Diagram is a tool that can be used to record different stages of the literature search process--across multiple resources--and clearly show how a researcher went from, 'These are the databases I searched for my terms', to, 'These are the papers I'm going to talk about'.
PRISMA is not inflexible; it can be modified to suit the research needs of different people and, indeed, if you did a Google images search for the flow diagram you would see many different versions of the diagram being used. It's a good idea to have a look at a couple of those examples, and also to have a look at a couple of the articles on the PRISMA website to see how it has--and can--be used.
The PRISMA 2020 Statement was published in 2021. It consists of a checklist and a flow diagram , and is intended to be accompanied by the PRISMA 2020 Explanation and Elaboration document.
In order to encourage dissemination of the PRISMA 2020 Statement, it has been published in several journals.
- How to use the PRISMA Flow Diagram for literature reviews A PDF [3.81MB] of the PowerPoint used to create the video. Each slide that has notes has a callout icon on the top right of the page which can be toggled on or off to make the notes visible.
There is also a PowerPoint version of the document and if you are a member of the University of Derby you can access the PRISMA Flow Diagram PPT via the link. (You will need to log in / be logged in with your University credentials to access this.)
This is an example of how you could fill in the PRISMA flow diagram when conducting a new review. It is not a hard and fast rule but it should give you an idea of how you can use it.
For more detailed information, please have a look at this article:
Page, M.J., McKenzie, J.E., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., Shamseer, L., Tetzlaff, J.M., Akl, E.A., Brennan, S.E., Chou, R., Glanville, J., Grimshaw, J.M., Hróbjartsson, A., Lalu, M.M., Li, T., Loder, E.W., Mayo-Wilson, E., McDonald, S., McGuinness, L.A., Stewart, L.A., Thomas, J., Tricco, A.C., Welch, V.A., Whiting,P. & Moher, D. (2021) 'The PRISMA 2020 statement: an updated guideline for reporting systematic reviews', BMJ 372:(71). doi: 10.1136/bmj.n71 .
- Example of PRISMA 2020 diagram This is an example of *one* of the PRISMA 2020 flow diagrams you can use when reporting on your research process. There is more than one form that you can use so for other forms and advice please look at the PRISMA website for full details.
Start using the flow diagram as you start searching the databases you've decided upon.
Make sure that you record the number of results that you found per database (before removing any duplicates) as per the filled in example. You can also do a Google images search for the PRISMA flow diagram to see the different ways in which people have used them to express their search processes.
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) PRISMA is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses. PRISMA focuses on the reporting of reviews evaluating randomized trials, but can also be used as a basis for reporting systematic reviews of other types of research, particularly evaluations of interventions.
- Prisma Flow Diagram This link will take you to downloadable Word and PDF copies of the flow diagram. These are modifiable and act as a starting point for you to record the process you engaged in from first search to the papers you ultimately discuss in your work. more... less... Do an image search on the internet for the flow diagram and you will be able to see all the different ways that people have modified the diagram to suit their personal research needs.
You can access the various checklists via the Equator website and the articles explaining PRISMA and its various extensions are available via PubMed.
Page, M.J., McKenzie, J.E., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., Shamseer, L., Tetzlaff, J.M., Akl, E.A., Brennan, S.E., Chou, R., Glanville, J., Grimshaw, J.M., Hróbjartsson, A., Lalu, M.M., Li, T., Loder, E.W., Mayo-Wilson, E., McDonald, S., McGuinness, L.A., Stewart, L.A., Thomas, J., Tricco, A.C., Welch, V.A., Whiting, P., & Moher, D. (2021) ' The PRISMA 2020 statement: an updated guideline for reporting systematic reviews,' BMJ . Mar 29; 372:n71. doi: 10.1136/bmj.n71 .
Page, M.J., Moher, D., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., Shamseer, L., Tetzlaff, J.M., Akl, E.A., Brennan, S.E., Chou, R., Glanville, J., Grimshaw, J.M., Hróbjartsson, A., Lalu, M.M., Li, T., Loder, E.W., Mayo-Wilson, E., McDonald, S., McGuinness, L.A., Stewart, L.A., Thomas, J., Tricco, A.C., Welch, V.A., Whiting, P., & McKenzie, J.E. (2021) 'PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews', BMJ, Mar 29; 372:n160. doi: 10.1136/bmj.n160 .
Page, M.J., McKenzie, J.E., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., Shamseer, L., Tetzlaff, J.M., Akl, E.A., Brennan, S.E., Chou, R., Glanville, J., Grimshaw, J.M., Hróbjartsson, A., Lalu, M.M., Li, T., Loder, E.W., Mayo-Wilson, E., McDonald, S., McGuinness, L.A., Stewart, L.A., Thomas, J., Tricco, A.C., Welch, V.A., Whiting, P., & Moher, D. (2021) ' The PRISMA 2020 statement: An updated guideline for reporting systematic reviews,' Journal of Clinical Epidemiology, June; 134:178-189. doi: 10.1016/j.jclinepi.2021.03.001 .
- << Previous: Exporting from different databases
- Next: Grey Literature >>
- Last Updated: Nov 19, 2024 12:51 PM
- URL: https://libguides.derby.ac.uk/literature-reviews

PRISMA Literature Review (Flow Chart & Example)
- Post author By admin
- Post date November 1, 2024
- No Comments on PRISMA Literature Review (Flow Chart & Example)
Are you planning to conduct a systematic literature review and want to follow the PRISMA protocol for this?
It’s easier than you think!
In this article, I’ll explain what PRISMA is and show you exactly how you can apply it in your own literature review.
What is a PRISMA Literature Review?
PRISMA stands for “Preferred Reporting Items for Systematic Reviews and Meta-Analyses.” It’s a guideline developed to improve the process and reporting of systematic reviews and meta-analyses.
These literature-based papers are particularly valuable because they summarize the findings of many individual studies, providing a more comprehensive picture of a topic.
The PRISMA guidelines offer a standardized framework that ensures all important aspects of a systematic review are reported transparently and completely. This includes describing the search strategy, the criteria for selecting studies, the method for data extraction, and the assessment of study quality.
One important point is that PRISMA does not provide specific instructions on how to conduct the systematic review itself.
It does not include detailed steps for what databases to select or, how to analyze the data. These tasks fall under the methodology of the systematic review and are a bit dependent on your field. Therefore, you need to come up with your own analysis method and combine it with PRISMA.
However, PRISMA helps guide you through the systematic search process step by step and documents it thoroughly.
The Goals of PRISMA
The main goals of PRISMA are:
- Transparency : Ensuring that your search strategy is clearly and thoroughly described so that other researchers can replicate and verify your study.
- Completeness : All relevant information must be reported to give readers a full picture of your literature search.
- Comparability : By standardizing the reporting, it becomes easier to compare and evaluate different systematic reviews.
You can find a complete overview here: https://www.prisma-statement.org/prisma-2020 .
When following the PRISMA guidelines, always make sure to cite the original source that contains the most recent version of the guidelines. The current version is PRISMA 2020. Here’s the complete reference for the PRISMA 2020 guidelines:
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., … & Moher, D. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021, 372.
What is the PRISMA Flow Chart?
The PRISMA flow chart, also sometimes called the PRISMA diagram, is a chart that shows how studies are selected for a systematic review.
It consists of four main phases:
- Identification : You search databases and other sources for studies and record the total number of studies found.
- Screening : You review the titles and abstracts of the studies and filter out those that are not relevant.
- Eligibility : You read the full text of the remaining studies and exclude those that do not fit your criteria.
- Inclusion : The final group of studies that will be included in your literature review or meta-analysis remains.
The PRISMA diagram helps you document the selection process clearly and ensures that nothing important is overlooked.
In the methods section of your paper, you should mention that your systematic review followed the PRISMA guidelines.
By explicitly mentioning PRISMA in the methodology section, you ensure that readers (and your supervisor) recognize and (hopefully) appreciate the structured approach of your systematic review.
Implementing a PRISMA Literature Search
Here are a few simple steps to implement the PRISMA literature search in your own work:
- Research : Search multiple databases, such as PubMed or Scopus, for relevant studies. Make a note of how and where you searched.
- Study Selection : Review the studies and remove those that don’t fit your criteria. Use the PRISMA diagram to document this process. You’ll need to develop your own selection criteria.
- Data Extraction : Gather key information from the selected studies, such as sample size, methods, and results. What exactly you extract depends on what you’re investigating.
- Study Quality Assessment : Assess the quality of the studies to ensure they are reliable.
Example of a Literature Review Using a PRISMA Diagram
To show you how PRISMA works in practice, let’s take a look at a paper that followed the PRISMA guidelines. The systematic review by Helen Crompton and Diane Burke, “Artificial intelligence in higher education: the state of the field,” examines the use of artificial intelligence (AI) in higher education.
The PRISMA guidelines were used in this study to make the process of the systematic review transparent and complete. Here’s a simple explanation of how the PRISMA guidelines were applied:
- Identification : The researchers conducted a literature search across several databases, identifying 341 relevant studies. Additionally, they conducted a manual search, finding 34 more studies. A manual search means that the researchers independently searched specific journals, reference lists, search engines, and websites in addition to the automated database search to ensure that no relevant studies were overlooked. Four duplicate studies were removed.
- Screening : After removing duplicates, 371 articles remained. After reviewing the titles and abstracts, no articles were excluded, so all 371 proceeded to full-text screening.
- Eligibility : The remaining articles were read in full and assessed. Some studies were excluded for the following reasons:
- No original research (n = 68): These articles were not original studies, but rather reviews or commentaries.
- Not in the field of higher education (n = 55): Studies were not related to higher education.
- No artificial intelligence (n = 92): These studies did not deal with AI.
- No use of AI for educational purposes (n = 18): AI was not used for educational purposes in these studies.
- Inclusion : Finally, 138 articles were included in the systematic review. These articles were analyzed in detail and qualitatively coded to answer the study’s research questions.
Source: Crompton, H., & Burke, D. (2023). Artificial intelligence in higher education: the state of the field . International Journal of Educational Technology in Higher Education, 20(1), 22.
You just need to fill out the PRISMA flowchart with the results of your literature search and screening, and you can include it in the methods section of your paper as a figure. Super easy, right?
The PRISMA Checklist
Additionally, PRISMA offers useful resources like a checklist, available on the PRISMA website. This checklist helps ensure that systematic reviews and meta-analyses are reported in a complete and transparent manner. It consists of 27 items, organized into different sections, and serves as a guide to structure your review.
This checklist is particularly relevant if you are preparing a full systematic review for your thesis or paper.
Checklist Summary:
- Title and Abstract : Clearly state that it is a systematic review. Provide a concise overview of the study.
- Introduction : Outline the background and reasons for the review. Clearly define the review’s objectives and research questions.
- Methods : Specify the inclusion and exclusion criteria for the studies. Describe the information sources and search strategies. Explain the selection and data extraction processes. Outline methods for assessing risk of bias and measures of effect. Detail how data from different studies were combined and analyzed.
- Results : Present the results of the search and selection process, ideally using a flow diagram. Summarize the characteristics and findings of the included studies. Evaluate the risk of bias and the certainty of the results.
- Discussion : Interpret the findings in the context of other evidence. Address the limitations of the evidence and methods. Consider the implications for practice and future research.
- Additional Information : Provide details on the registration and protocol of the review. List both financial and non-financial sources of support. Disclose any potential conflicts of interest among the authors. Indicate the availability of data and materials.
While other PRISMA resources may be useful for high-level publications or complex meta-analyses, for your studies, the most relevant parts are the flowchart and sections of the checklist.
If you have any questions, feel free to leave a comment!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

Welcome to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) website
Here you can access information about the PRISMA reporting guidelines, which are designed to help authors transparently report why their systematic review was done, what methods they used, and what they found.
The main PRISMA reporting guideline (the PRISMA 2020 statement) primarily provides guidance for the reporting of systematic reviews evaluating the effects of interventions. PRISMA 2020 is complemented by various PRISMA extensions , which provide guidance for the reporting of different types or aspects of systematic reviews and other types of evidence synthesis (e.g. scoping reviews).
Development, updating and implementation of the PRISMA reporting guidelines is overseen by the PRISMA Executive , which is currently co-chaired by Prof Joanne McKenzie and Dr Matthew Page at Monash University.
Key documents
PRISMA 2020 checklist
PRISMA 2020 flow diagram
PRISMA 2020 statement paper
PRISMA 2020 Explanation and Elaboration paper

The browser you are using is no longer supported and for that reason you will not get the best experience when using our website.
You currently have JavaScript disabled in your web browser, please enable JavaScript to view our website as intended.
In this section
- Registering
- Return to the start of the menu
Library and Learning Services
- Research support
What is PRISMA, and why do you need a protocol?
PRISMA stands for Preferred Reporting Items for Systematic reviews and Meta-Analyses.
If you are planning on publishing your systematic review then you will want to follow the PRISMA guidelines and checklists, as this is the standard format for reporting systematic reviews.
For example, if you have read any systematic reviews you will have seen they include items like the PRISMA flow diagram. It is useful to be familiar with PRISMA before you write your protocol and embark on your review. The following resources may be helpful:
- PRISMA website
- The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, BMJ 2021; 372. doi:10.1136/bmj.n71 .
- PRISMA 2020 checklist (docx, 30.2kb)
- PRISMA 2020 flow diagram .
You may wish to use a spreadsheet, such as the one provided by the Cochrane Airways Group , to record items you will need to complete your PRISMA flow diagram. We highly recommend documenting all stages of your review to help with reporting.
What is a protocol?
Once you have decided that your systematic review is viable then you need to write a protocol. This is a plan of how you will conduct your review. It will make it easier to understand what needs to be done at each stage of your review.
At this point you may decide to refine your research question further and break it down using one of the common frameworks available. These frameworks can also help you create your search strategy.
For example, the most common framework used in health reviews is PICO:
- Population/Problem
- Intervention or Issue
- Comparison (if any)
- Outcomes.
See our guide on Planning a search using PICO (PDF, 185kb) .
There are other frameworks available and you should pick the one most useful to you. In some subject areas there may not be a relevant framework to use.
Other frameworks
- PICO variants = PICOT (T is time within which the outcome will be observed, or study type) and PICOS (S is study type).
- PECO = Population, Exposure, Comparison, Outcome.
- PIRT = Population, Index Test, Reference Test, Target Condition.
- SPIDER = Sample, Phenomenon of Interest, Design, Evaluation, Research Type.
- PCC = Population/Problem, Concept, Context (for Scoping Reviews).
Inclusion and exclusion criteria
When you are writing your protocol and thinking about your search strategy you will need to define your inclusion and exclusion criteria. This is the criteria you will use to decide if a study is eligible to be included in your review.
Examples of inclusion/exclusion criteria
- Study design – what type of studies are you including in your review? Quantitative, qualitative or both? Are you only going to include specific study types e.g. randomised controlled trials?
- Population – are you looking at a specific population e.g. by age, gender, ethnicity or geography?
- Years of publication – are you limiting your review to a certain range of years e.g. the last ten years? It is advisable to have a robust reason for limiting by year. You might be updating an older review; a guideline or treatment came into effect from that date etc.
- English language only – many reviews do limit to English language only publications for practical reasons, but if you do this you must acknowledge it is a weakness in your review.
Further resources
- Webinar from the Centre for Open Science on developing inclusion/exclusion criteria: Screening for studies in systematic reviews, scoping reviews, and other knowledge syntheses: Strategies for improvement (April 2020).
- Chapter 3 of the Cochrane Handbook: Defining the criteria for including studies and how they will be grouped for the synthesis .
- PRISMA for systematic review protocols (PRISMA-P) .
- Open access
- Published: 19 April 2021
How to properly use the PRISMA Statement
- Rafael Sarkis-Onofre 1 ,
- Ferrán Catalá-López 2 , 3 ,
- Edoardo Aromataris 4 &
- Craig Lockwood 4
Systematic Reviews volume 10 , Article number: 117 ( 2021 ) Cite this article
94k Accesses
321 Citations
94 Altmetric
Metrics details
A Research to this article was published on 29 March 2021
It has been more than a decade since the original publication of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [ 1 ], and it has become one of the most cited reporting guidelines in biomedical literature [ 2 , 3 ]. Since its publication, multiple extensions of the PRISMA Statement have been published concomitant with the advancement of knowledge synthesis methods [ 4 , 5 , 6 , 7 ]. The PRISMA2020 statement, an updated version has recently been published [ 8 ], and other extensions are currently in development [ 9 ].
The number of systematic reviews (SRs) has increased substantially over the past 20 years [ 10 , 11 , 12 ]. However, many SRs continue to be poorly conducted and reported [ 10 , 11 ], and it is still common to see articles that use the PRISMA Statement and other reporting guidelines inappropriately, as was highlighted recently [ 13 ].
The PRISMA Statement and its extensions are an evidence-based, minimum set of recommendations designed primarily to encourage transparent and complete reporting of SRs. This growing set of guidelines have been developed to aid authors with appropriate reporting of different knowledge synthesis methods (such as SRs, scoping reviews, and review protocols) and to ensure that all aspects of this type of research are accurately and transparently reported. In other words, the PRISMA Statement is a road map to help authors best describe what was done, what was found, and in the case of a review protocol, what are they are planning to do.
Despite this clear and well-articulated intention [ 2 , 3 , 4 , 5 ], it is common for Systematic Reviews to receive manuscripts detailing the inappropriate use of the PRISMA Statement and its extensions. Most frequently, improper use appears with authors attempting to use the PRISMA statement as a methodological guideline for the design and conduct reviews, or identifying the PRISMA statement as a tool to assess the methodological quality of reviews, as seen in the following examples:
“This scoping review will be conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Statement.”
“This protocol was designed based on the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) Statement.”
“The methodological quality of the included systematic reviews will be assessed with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement.”
Some organizations (such as Cochrane and JBI) have developed methodological guidelines that can help authors to design or conduct diverse types of knowledge synthesis rigorously [ 14 , 15 ]. While the PRISMA statement is presented to predominantly guide reporting of a systematic review of interventions with meta-analyses, its detailed criteria can readily be applied to the majority of review types [ 13 ]. Differences between the role of the PRISMA Statement to guide reporting versus guidelines detailing methodological conduct is readily illustrated with the following example: the PRISMA Statement recommends that authors report their complete search strategies for all databases, registers, and websites (including any filters and limits used), but it does not include recommendations for designing and conducting literature searches [ 8 ]. If authors are interested in understanding how to create search strategies or which databases to include, they should refer to the methodological guidelines [ 12 , 13 ]. Thus, the following examples can illustrate the appropriate use of the PRISMA Statement in research reporting:
“The reporting of this systematic review was guided by the standards of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) Statement.”
“This scoping review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR).”
“The protocol is being reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) Statement.”
Systematic Reviews supports the complete and transparent reporting of research. The Editors require the submission of a populated checklist from the relevant reporting guidelines, including the PRISMA checklist or the most appropriate PRISMA extension. Using the PRISMA statement and its extensions to write protocols or the completed review report, and completing the PRISMA checklists are likely to let reviewers and readers know what authors did and found, but also to optimize the quality of reporting and make the peer review process more efficient.
Transparent and complete reporting is an essential component of “good research”; it allows readers to judge key issues regarding the conduct of research and its trustworthiness and is also critical to establish a study’s replicability.
With the release of a major update to PRISMA in 2021, the appropriate use of the updated PRISMA Statement (and its extensions as those updates progress) will be an essential requirement for review based submissions, and we encourage authors, peer reviewers, and readers of Systematic Reviews to use and disseminate that initiative.
Availability of data and materials
We do not have any additional data or materials to share.

Abbreviations
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews
Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols
Systematic reviews
Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. https://doi.org/10.1016/j.jclinepi.2009.06.005 .
Article PubMed Google Scholar
Caulley L, Cheng W, Catala-Lopez F, Whelan J, Khoury M, Ferraro J, et al. Citation impact was highly variable for reporting guidelines of health research: a citation analysis. J Clin Epidemiol. 2020;127:96–104. https://doi.org/10.1016/j.jclinepi.2020.07.013 .
Page MJ, Moher D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and extensions: a scoping review. Syst Rev. 2017;6(1):263. https://doi.org/10.1186/s13643-017-0663-8 .
Article PubMed PubMed Central Google Scholar
Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, et al. PRISMA-S: an extension to the PRISMA Statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10(1):39. https://doi.org/10.1186/s13643-020-01542-z .
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–73. https://doi.org/10.7326/M18-0850 .
Article Google Scholar
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. https://doi.org/10.1186/2046-4053-4-1 .
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. https://doi.org/10.7326/M14-2385 .
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. https://doi/10.1186/s13643-021-01626-4.
EQUATOR Network: Reporting guidelines under development for systematic reviews. https://www.equator-network.org/library/reporting-guidelines-under-development/reporting-guidelines-under-development-for-systematic-reviews/ . Accessed 11 Feb 2021.
Page MJ, Shamseer L, Altman DG, Tetzlaff J, Sampson M, Tricco AC, et al. Epidemiology and Reporting Characteristics of Systematic Reviews of Biomedical Research: A Cross-Sectional Study. Plos Med. 2016;13(5):e1002028. https://doi.org/10.1371/journal.pmed.1002028 .
Ioannidis JP. The Mass Production of Redundant, Misleading, and Conflicted Systematic Reviews and Meta-analyses. Milbank Q. 2016;94(3):485–514. https://doi.org/10.1111/1468-0009.12210 .
Niforatos JD, Weaver M, Johansen ME. Assessment of Publication Trends of Systematic Reviews and Randomized Clinical Trials, 1995 to 2017. JAMA Intern Med. 2019;179(11):1593–4. https://doi.org/10.1001/jamainternmed.2019.3013.
Caulley L, Catala-Lopez F, Whelan J, Khoury M, Ferraro J, Cheng W, et al. Reporting guidelines of health research studies are frequently used inappropriately. J Clin Epidemiol. 2020;122:87–94. https://doi.org/10.1016/j.jclinepi.2020.03.006 .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition ed. Chichester: Wiley; 2019.
Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. ed. Adelaide: JBI; 2020.
Download references
Acknowledgements
RSO is funded in part by Meridional Foundation. FCL is funded in part by the Institute of Health Carlos III/CIBERSAM.
Author information
Authors and affiliations.
Graduate Program in Dentistry, Meridional Faculty, IMED, Passo Fundo, Brazil
Rafael Sarkis-Onofre
Department of Health Planning and Economics, National School of Public Health, Institute of Health Carlos III, Madrid, Spain
Ferrán Catalá-López
Department of Medicine, University of Valencia/INCLIVA Health Research Institute and CIBERSAM, Valencia, Spain
JBI, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, Australia
Edoardo Aromataris & Craig Lockwood
You can also search for this author in PubMed Google Scholar
Contributions
RSO drafted the initial version. FCL, EA, and CL made substantial additions to the first and subsequent drafts. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Rafael Sarkis-Onofre .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
CL is Editor-in-Chief of Systematic Reviews, FCL is Protocol Editor of Systematic Reviews, and RSO is Associate Editor of Systematic Reviews.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sarkis-Onofre, R., Catalá-López, F., Aromataris, E. et al. How to properly use the PRISMA Statement. Syst Rev 10 , 117 (2021). https://doi.org/10.1186/s13643-021-01671-z
Download citation
Published : 19 April 2021
DOI : https://doi.org/10.1186/s13643-021-01671-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

How to properly use the PRISMA Statement
Rafael sarkis-onofre, ferrán catalá-lópez, edoardo aromataris, craig lockwood.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Collection date 2021.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
It has been more than a decade since the original publication of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [ 1 ], and it has become one of the most cited reporting guidelines in biomedical literature [ 2 , 3 ]. Since its publication, multiple extensions of the PRISMA Statement have been published concomitant with the advancement of knowledge synthesis methods [ 4 – 7 ]. The PRISMA2020 statement, an updated version has recently been published [ 8 ], and other extensions are currently in development [ 9 ].
The number of systematic reviews (SRs) has increased substantially over the past 20 years [ 10 – 12 ]. However, many SRs continue to be poorly conducted and reported [ 10 , 11 ], and it is still common to see articles that use the PRISMA Statement and other reporting guidelines inappropriately, as was highlighted recently [ 13 ].
The PRISMA Statement and its extensions are an evidence-based, minimum set of recommendations designed primarily to encourage transparent and complete reporting of SRs. This growing set of guidelines have been developed to aid authors with appropriate reporting of different knowledge synthesis methods (such as SRs, scoping reviews, and review protocols) and to ensure that all aspects of this type of research are accurately and transparently reported. In other words, the PRISMA Statement is a road map to help authors best describe what was done, what was found, and in the case of a review protocol, what are they are planning to do.
Despite this clear and well-articulated intention [ 2 – 5 ], it is common for Systematic Reviews to receive manuscripts detailing the inappropriate use of the PRISMA Statement and its extensions. Most frequently, improper use appears with authors attempting to use the PRISMA statement as a methodological guideline for the design and conduct reviews, or identifying the PRISMA statement as a tool to assess the methodological quality of reviews, as seen in the following examples:
“This scoping review will be conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Statement.”
“This protocol was designed based on the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) Statement.”
“The methodological quality of the included systematic reviews will be assessed with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement.”
Some organizations (such as Cochrane and JBI) have developed methodological guidelines that can help authors to design or conduct diverse types of knowledge synthesis rigorously [ 14 , 15 ]. While the PRISMA statement is presented to predominantly guide reporting of a systematic review of interventions with meta-analyses, its detailed criteria can readily be applied to the majority of review types [ 13 ]. Differences between the role of the PRISMA Statement to guide reporting versus guidelines detailing methodological conduct is readily illustrated with the following example: the PRISMA Statement recommends that authors report their complete search strategies for all databases, registers, and websites (including any filters and limits used), but it does not include recommendations for designing and conducting literature searches [ 8 ]. If authors are interested in understanding how to create search strategies or which databases to include, they should refer to the methodological guidelines [ 12 , 13 ]. Thus, the following examples can illustrate the appropriate use of the PRISMA Statement in research reporting:
“The reporting of this systematic review was guided by the standards of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) Statement.”
“This scoping review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR).”
“The protocol is being reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) Statement.”
Systematic Reviews supports the complete and transparent reporting of research. The Editors require the submission of a populated checklist from the relevant reporting guidelines, including the PRISMA checklist or the most appropriate PRISMA extension. Using the PRISMA statement and its extensions to write protocols or the completed review report, and completing the PRISMA checklists are likely to let reviewers and readers know what authors did and found, but also to optimize the quality of reporting and make the peer review process more efficient.
Transparent and complete reporting is an essential component of “good research”; it allows readers to judge key issues regarding the conduct of research and its trustworthiness and is also critical to establish a study’s replicability.
With the release of a major update to PRISMA in 2021, the appropriate use of the updated PRISMA Statement (and its extensions as those updates progress) will be an essential requirement for review based submissions, and we encourage authors, peer reviewers, and readers of Systematic Reviews to use and disseminate that initiative.
Acknowledgements
Abbreviations.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews
Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols
Systematic reviews
Authors’ contributions
RSO drafted the initial version. FCL, EA, and CL made substantial additions to the first and subsequent drafts. All authors read and approved the final manuscript.
RSO is funded in part by Meridional Foundation. FCL is funded in part by the Institute of Health Carlos III/CIBERSAM.
Availability of data and materials
We do not have any additional data or materials to share.
Declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
CL is Editor-in-Chief of Systematic Reviews, FCL is Protocol Editor of Systematic Reviews, and RSO is Associate Editor of Systematic Reviews.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- 1. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Caulley L, Cheng W, Catala-Lopez F, Whelan J, Khoury M, Ferraro J, et al. Citation impact was highly variable for reporting guidelines of health research: a citation analysis. J Clin Epidemiol. 2020;127:96–104. doi: 10.1016/j.jclinepi.2020.07.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Page MJ, Moher D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and extensions: a scoping review. Syst Rev. 2017;6(1):263. doi: 10.1186/s13643-017-0663-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, et al. PRISMA-S: an extension to the PRISMA Statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10(1):39. doi: 10.1186/s13643-020-01542-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JPA, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. https://doi/10.1186/s13643-021-01626-4. [ DOI ] [ PMC free article ] [ PubMed ]
- 9. EQUATOR Network: Reporting guidelines under development for systematic reviews. https://www.equator-network.org/library/reporting-guidelines-under-development/reporting-guidelines-under-development-for-systematic-reviews/ . Accessed 11 Feb 2021.
- 10. Page MJ, Shamseer L, Altman DG, Tetzlaff J, Sampson M, Tricco AC, Catalá-López F, Li L, Reid EK, Sarkis-Onofre R, Moher D. Epidemiology and Reporting Characteristics of Systematic Reviews of Biomedical Research: A Cross-Sectional Study. Plos Med. 2016;13(5):e1002028. doi: 10.1371/journal.pmed.1002028. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Ioannidis JP. The Mass Production of Redundant, Misleading, and Conflicted Systematic Reviews and Meta-analyses. Milbank Q. 2016;94(3):485–514. doi: 10.1111/1468-0009.12210. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Niforatos JD, Weaver M, Johansen ME. Assessment of Publication Trends of Systematic Reviews and Randomized Clinical Trials, 1995 to 2017. JAMA Intern Med. 2019;179(11):1593–4. https://doi.org/10.1001/jamainternmed.2019.3013. [ DOI ] [ PMC free article ] [ PubMed ]
- 13. Caulley L, Catala-Lopez F, Whelan J, Khoury M, Ferraro J, Cheng W, et al. Reporting guidelines of health research studies are frequently used inappropriately. J Clin Epidemiol. 2020;122:87–94. doi: 10.1016/j.jclinepi.2020.03.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition ed. Chichester: Wiley; 2019.
- 15. Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. ed. Adelaide: JBI; 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
- View on publisher site
- PDF (450.2 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
COMMENTS
Nov 19, 2024 · The PRISMA Flow Diagram is a tool that can be used to record different stages of the literature search process--across multiple resources--and clearly show how a researcher went from, 'These are the databases I searched for my terms', to, 'These are the papers I'm going to talk about'.
Nov 1, 2024 · What is a PRISMA Literature Review? PRISMA stands for “Preferred Reporting Items for Systematic Reviews and Meta-Analyses.” It’s a guideline developed to improve the process and reporting of systematic reviews and meta-analyses.
The main PRISMA reporting guideline (the PRISMA 2020 statement) primarily provides guidance for the reporting of systematic reviews evaluating the effects of interventions.
PRISMA stands for Preferred Reporting Items for Systematic reviews and Meta-Analyses. If you are planning on publishing your systematic review then you will want to follow the PRISMA guidelines and checklists, as this is the standard format for reporting systematic reviews.
Apr 19, 2021 · The PRISMA Statement and its extensions are an evidence-based, minimum set of recommendations designed primarily to encourage transparent and complete reporting of SRs.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) is an evidence-based minimum set of items aimed at helping scientific authors to report a wide array of systematic reviews and meta-analyses, primarily used to assess the benefits and harms of a health care intervention.
The PRISMA Statement and its extensions are an evidence-based, minimum set of recommendations designed primarily to encourage transparent and complete reporting of SRs.