- 0 Shopping Cart 0.00 € -->


In Vitro Pharmacology for Drug Discovery & Development
- Share on WhatsApp
- Share on LinkedIn
- Share by Mail

WHAT YOU WILL LEARN IN THIS ARTICLE: What Is In Vitro Pharmacology? What Is Its Role in the Drug Discovery & Development Cycle? In Vitro Pharmacology in Drug Discovery In Vitro Pharmacology in Pre-clinical Trials Can We Fully Replace Animal Testing?
The commercialization of a new drug or medicine takes an average of 10 to 15 years. It is indeed an incredibly costly and complex process that involves multiple rounds of assays and tests. This is because bringing a new drug to market is extremely regulated by dedicated agencies (the EMA in the European Union and the FDA in the United States, just to mention some examples).
The considerations of efficacy and safety must be taken into account throughout the entire drug development process, from the screening of candidate molecules and the identification of the lead compound until the very last stage of pre-clinical trials. Thus, to generate high-quality data on the safety and efficacy of their drug candidates, researchers very often take advantage of in vitro pharmacology .
In this article, you will learn about the role of in vitro pharmacology studies at the different stages of drug discovery and development.
What Is In Vitro Pharmacology?
To understand the concept of in vitro pharmacology it is usually useful to split it in two:
in vitro , which refers to studies or experiments conducted on microorganisms and cells outside of their normal biological environment
pharmacology , which is the study of the effects of drugs and pharmaceuticals on living organisms
Putting them together, it is now easy to see that in vitro pharmacology is nothing other than the research of the biological effects of drugs and pharmaceuticals, conducted outside of living organisms (in opposition to research performed on living animals or humans).
But what is the purpose of these studies? What information do they generate? How do they help the pharmaceutical field? To answer these questions, we first need to get familiar with the drug discovery and development process, i.e., to understand how drugs are created, to see next where the in vitro pharmacology fits in that process.
What Is Its Role in the Drug Discovery and Development Cycles?
Any new drug or pharmaceutical that enters the market is the result of long and rigorous development, testing, and approval processes .
After the COVID-19 Pandemic, many people became familiar with the different phases of clinical trials, in which new drugs are tested in humans to collect data on drug safety and efficacy. However, the long and bumpy road that precedes it, which takes from the identification of a drug candidate to its production for clinical evaluation, is usually less known.
In Vitro Pharmacology in Drug Discovery
Before drug development comes drug discovery , the process by which new candidate drugs are identified .
Target identification
Fundamental science helps researchers understand how a disease or condition starts and progresses, thus making it possible to conceive new ways of interfering with these pathological processes. This is done by identifying therapeutical targets (a cellular structure, a surface receptor, or a transcription factor, just to name a few examples) against which the drug candidates are expected to act, both selectively and effectively.
Hit discovery
Target identification is then followed by the screening of large libraries of compounds to find a pool of drug candidates with the desired properties (or “ hits ”). As a result of additional tests, the pool is further narrowed down, and only the most promising compounds (or “ leads ”) are selected.
In silico screening approaches are a valuable tool since they help speed up this stage and save resources. Yet, in vitro assays are for the time being still necessary to validate bioinformatic predictions.
Lead optimization
Following the identification of leads, a great amount of effort is devoted to significantly optimizing numerous properties of the leads, including their biological activity. As expected, this involves the iterative work of chemists who design, synthesize, and characterize new compounds . So as data is gathered after each round of tests, a better picture of how the chemical structure and activity are related is built up, fueling optimization.
Similar to what occurs during the Hit Discovery phase, in vitro pharmacology assays are used to generate high-quality data that provides precise information on the biological properties of the compounds tested .
The lead optimization phase culminates in the identification of one or more drug candidates to be assessed during the pre-clinical trials.
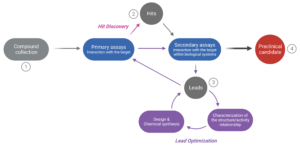
Figure 1 . The drug discovery cycle . The path from a large collection of potentially efficacious compounds (1) to selecting one or more preclinical candidates (4) involves several steps. In the “Hit Discovery” phase, numerous molecules are screened to evaluate their interaction with the target (primary assays). Yet, only a small number (the “hits”) will be selected for further evaluation (2). Their interaction with the target in a biological context is assessed (secondary assays involving in vitro cell cultures or animal testing) to narrow down the pool of hits. The most promising compounds (the “leads”) are selected for optimization during the “Lead Optimization” phase (3). At this stage, the leads are characterized at the structure and activity level, which helps improve the design and chemical synthesis of new but structurally-related compounds. The new compounds are re-assessed through primary and secondary assays, despite their assumed functional similarity. This iterative process continues until a compound yielding satisfactory results is identified (selection of a “Preclinical candidate”).
In Vitro Pharmacology in Pre-Clinical Trials
On average, only 1 in 5,000 compounds that enter pre-clinical trials obtains approval for commercialization. The reason for this is that when a drug candidate enters pre-clinical trials, there is not yet enough information regarding the compound’s safety, toxicity, pharmacokinetics, and metabolism . Drug licensing authorities require the submission of a body of pre-clinical data on all of these parameters before testing any new drug on humans. Thus, they are assessed at the pre-clinical stage, typically through both in vitro and in vivo studies. The dose for a drug’s first use in clinical trials is also determined at this step.
At the pre-clinical trial stage, in vitro pharmacology assays can be used for many different purposes:
- profile compounds to guide pre-clinical in vivo safety and toxicity studies
- obtain high-quality data on the safety and toxicity of a drug candidate
- identify potential adverse effects early in the drug development process
- assess the potency and efficacy of a drug candidate against the target disease or condition
- gather data on pharmacokinetics and pharmacodynamics
- study the drug candidate’s mechanism of action in more depth
- evaluate the activity of biosimilar compounds
As we can observe, safety pharmacology is a crucial stage of the pre-clinical development process. Its purpose is to assess any potential undesirable effects of the drug on the body’s major systems. Although most safety testing was conducted on animals in the past, safety tests are currently making increasing use of in vitro cell and tissue models.
Can We Fully Replace Animal Testing?
The countless applications of in vitro pharmacology tests and assays lead to a fair question concerning drug development: Could in vitro pharmacology fully replace animal testing in the future?
Unfortunately, although it’s the case for the cosmetics and personal care industry, non-animal alternatives aren’t currently sufficient for safety and efficacy testing in the pharmaceutical industry . Due to extremely rigorous demands, animal testing is still used to investigate repeat-dose toxicity, carcinogenicity, and several other properties.
However, the ongoing pursuit of better animal welfare keeps driving the improvement and development of new in vitro technologies, so a future without animal testing is a realistic possibility.
Deore A. B. et al. The Stages of Drug Discovery and Development Process. Asian Journal of Pharmaceutical Research and Development (2019).
Hughes J. P. et al. Principles of early drug discovery. British Journal of Pharmacology (2010).
The Drug Development Process, US Food & Drug Administration (FDA), accessed 02 March 2023.
Find an In Vitro Pharmacology Program to Suit Your Needs
At QIMA Life Sciences , we are committed to developing effective in vitro alternatives to animal experimentation methods. For this, we offer a comprehensive selection of services to support your research needs in biology and in vitro pharmacology.
Our scientists have extensive expertise and know-how that can be applied to a wide range of pre-clinical and clinical research projects . We offer customized technical solutions that can best meet your requirements, prerequisites, and objectives, with particular attention given to the feasibility study and personalized management of your projects. With our state-of-the-art facilities and over 20 years of experience supporting projects in the pharmaceutical and biotechnological industries, we are pleased to offer you dedicated project management support and consulting for your R&D process.
Ready to discuss a testing and research program that will suit the needs of your business?
Get in touch with the QIMA Life Sciences team today!
You may also be interested in:
In vitro pharmacological solutions for psoriasis.

In vitro pharmacological solutions for WOUND CARE

Pharmacological solutions for ATOPIC DERMATITIS

Cosmetics & Personal Care
- Skin barrier and hydration
- Skin ageing
- Oily skin and hyperseborrhea
- Skin pigmentation
- Skin microbiota
- Hair Growth
- Hair Microbiota
- Hair Pigmentation
Pharma & Biotech
- Atopic dermatitis
- Wound healing and skin regeneration
- Immune-inflammation
- Neurobiology
- Veterinary medicine
NUTRACEUTICALS
France QIMA Bioalternatives: +33 (0)5 49 36 11 37 (Gençay) +33 (0)5 61 28 71 60 (Labège)
QIMA Newtone: +33 (0)4 72 69 83 20
Germany QIMA Monasterium: +49 (0) 251 93264458
Forgot password ?
Lost your password? Please enter your email address. You will receive mail with link to set new password.
Retour connexion
Privacy Overview
Documentary resources_poster_ifscc 2024_downloading-ai.
- Surname * First Last
- Country * Country Afghanistan Albania Algeria American Samoa Andorra Angola Anguilla Antarctica Antigua and Barbuda Argentina Armenia Aruba Australia Austria Azerbaijan Bahamas Bahrain Bangladesh Barbados Belarus Belgium Belize Benin Bermuda Bhutan Bolivia Bonaire, Sint Eustatius and Saba Bosnia and Herzegovina Botswana Bouvet Island Brazil British Indian Ocean Territory Brunei Darussalam Bulgaria Burkina Faso Burundi Cabo Verde Cambodia Cameroon Canada Cayman Islands Central African Republic Chad Chile China Christmas Island Cocos Islands Colombia Comoros Congo Congo, Democratic Republic of the Cook Islands Costa Rica Croatia Cuba Curaçao Cyprus Czechia Côte d'Ivoire Denmark Djibouti Dominica Dominican Republic Ecuador Egypt El Salvador Equatorial Guinea Eritrea Estonia Eswatini Ethiopia Falkland Islands Faroe Islands Fiji Finland France French Guiana French Polynesia French Southern Territories Gabon Gambia Georgia Germany Ghana Gibraltar Greece Greenland Grenada Guadeloupe Guam Guatemala Guernsey Guinea Guinea-Bissau Guyana Haiti Heard Island and McDonald Islands Holy See Honduras Hong Kong Hungary Iceland India Indonesia Iran Iraq Ireland Isle of Man Israel Italy Jamaica Japan Jersey Jordan Kazakhstan Kenya Kiribati Korea, Democratic People's Republic of Korea, Republic of Kuwait Kyrgyzstan Lao People's Democratic Republic Latvia Lebanon Lesotho Liberia Libya Liechtenstein Lithuania Luxembourg Macao Madagascar Malawi Malaysia Maldives Mali Malta Marshall Islands Martinique Mauritania Mauritius Mayotte Mexico Micronesia Moldova Monaco Mongolia Montenegro Montserrat Morocco Mozambique Myanmar Namibia Nauru Nepal Netherlands New Caledonia New Zealand Nicaragua Niger Nigeria Niue Norfolk Island North Macedonia Northern Mariana Islands Norway Oman Pakistan Palau Palestine, State of Panama Papua New Guinea Paraguay Peru Philippines Pitcairn Poland Portugal Puerto Rico Qatar Romania Russian Federation Rwanda Réunion Saint Barthélemy Saint Helena, Ascension and Tristan da Cunha Saint Kitts and Nevis Saint Lucia Saint Martin Saint Pierre and Miquelon Saint Vincent and the Grenadines Samoa San Marino Sao Tome and Principe Saudi Arabia Senegal Serbia Seychelles Sierra Leone Singapore Sint Maarten Slovakia Slovenia Solomon Islands Somalia South Africa South Georgia and the South Sandwich Islands South Sudan Spain Sri Lanka Sudan Suriname Svalbard and Jan Mayen Sweden Switzerland Syria Arab Republic Taiwan Tajikistan Tanzania, the United Republic of Thailand Timor-Leste Togo Tokelau Tonga Trinidad and Tobago Tunisia Turkmenistan Turks and Caicos Islands Tuvalu Türkiye US Minor Outlying Islands Uganda Ukraine United Arab Emirates United Kingdom United States Uruguay Uzbekistan Vanuatu Venezuela Viet Nam Virgin Islands, British Virgin Islands, U.S. Wallis and Futuna Western Sahara Yemen Zambia Zimbabwe Åland Islands Country
- Keep up with what’s happening in QIMA Life Sciences!
- I have read and accept the Terms of Service of qima-lifesciences.com
Les informations recueillies sur ce formulaire sont enregistrées dans un fichier informatisé par QIMA LIFE SCIENCES pour vous contacter afin de répondre à votre demande et vous aider à sélectionner le ou les tests les mieux adaptés à votre projet.
La base légale du traitement est l’intérêt légitime du QIMA Life Sciences. Les données collectées seront communiquées aux seuls destinataires suivants : Les services concernés et habilités par QIMA Life Sciences. Les données sont conservées pendant une durée de 3 ans à compter de la collecte ou de votre dernier contact entrant.
Vous pouvez accéder aux données vous concernant, les rectifier, demander leur effacement ou exercer votre droit à la limitation du traitement de vos données, retirer votre consentement au traitement de vos données à tout moment, vous opposer au traitement de vos données et vous disposez d’un droit à la portabilité de vos données.
Consultez le site cnil.fr pour plus d’informations sur vos droits.
Pour exercer ces droits vous pouvez nous contacter à l’aide du lien suivant Gestion des droits ou en écrivant à [email protected] .
Si vous estimez, après nous avoir contactés, que vos droits « Informatique et Libertés » ne sont pas respectés, vous pouvez adresser une réclamation à la CNIL.
N.B : Les catégories de données suivantes : Email / Pays / Société / Sélectionnez la vidéo sont obligatoires. La non-fourniture de ces informations entrainera l’impossibilité pour QIMA Life Sciences de vous répondre.
- Phone This field is for validation purposes and should be left unchanged.
Documentary Resources_Poster_IFSCC 2024_Downloading
- Name This field is for validation purposes and should be left unchanged.
Documentary Resources_Flyer_Vitiligo_Downloading
Documentary resources_slide deck_eadv2024_wound healing transcriptomic profiling_downloading, documentary resources_technical sheet_dynacam.
- Comments This field is for validation purposes and should be left unchanged.
Documentary Resources_Webinar Summaries_Longevity & aging_Access Request
Documentary resources_flyer_make up_downloading, documentary resources_flyer_hair graying_downloading, documentary resources_flyer_clinical imaging for veterinary applications_downloading, documentary resources_short video_vitil-ia_access request, documentary resources_short video_skincam pro_access request, documentary resources_poster_embrn 2024_downloading.
- Email This field is for validation purposes and should be left unchanged.

Documentary Resources_Poster_SID 2024_Downloading_
Documentary resources_short video_seboregulation_access request, documentary resources_poster_sid 2024_downloading, documentary resources_slide deck_digicam_downloading, documentary resources_webinar summaries_immunology testing-pharma_access request, documentary resources_flyer_hair growth_downloading, documentary resources_short video_veterinary services_access request, documentary resources_short video_hair follicle & skin microbiome_access request, documentary resources_poster_aad 2024_downloading, documentary resources_flyer_sebocyte cell line_downloading, documentary resources_webinar summaries_models for hair research and testing_access request, documentary resources_poster_ehsf 2024_downloading, documentary resources_flyer_hair skinification_downloading, documentary resources_poster_iuis 2023_downloading, documentary resources_flyer_cellular senescence & skin aging_downloading, documentary resources_flyer_mast cells_downloading, documentary resources_flyer_mitochondria_downloading, documentary resources_technical sheet_skincampro_downloading, documentary resources_flyer_human skin explants_downloading, documentary resources_poster_cosm'innov-2023_downloading, documentary resources_poster_ifscc 2023'_downloading, documentary resources_poster_ifscc 2023_downloading, documentary resources_technical sheet_spectraface_downloading, documentary resources_technical sheet_spectracam_downloading, documentary resources_technical sheet_eyelashcam_downloading, documentary resources_webinar summaries_models for atopic dermatitis_access request, documentary resources_short video_healthcare and pharmacology_access request, documentary resources_technical sheet_skincam_downloading, documentary resources_flyer_immuno-oncology assays_downloading, documentary resources_technical sheet_colorface_downloading, documentary resources_short video_oncology-related assays_access request, documentary resources_flyer_assesment of compound allergenicity_downloading, documentary resources_flyer_ceramides_downloading, documentary resources_webinar summaries_immunology testing_access request, documentary resources_flyer_skin barrier & hydration_downloading, documentary resources_webinar summaries_skin barrier & hydration_access request, documentary resources_webinar summaries_skin microbiota and consequences of dysbiosis_access request, documentary resources_application notes_psoriasis_access request, documentary resources_application notes_hair pigmentation & canities_access request, documentary resources_application notes_wound healing_access request, documentary resources_application notes_atopic dermatitis_access request, documentary resources_short video_skin biosense in tubo_access request, documentary resources_short video_skin biosense in vitro_access request, documentary resources.
- Type of resource * Webinar summaries Application note Presentations
- Select the document * Immune response testing Skin tissue engineering Skin barrier & Hydration Bioanalysis of non-invasive clinical samples Skin microbiota & consequences of dysbiosis
- Select the document * Psoriasis therapies Hair pigmentation & canities Acne vulgaris Wound care therapies Hair growth & regeneration Atopic Dermatitis
- Select the document * Newtone_Digital Gradin Tool
N.B : Les catégories de données suivantes : Email / Pays sont obligatoires. La non-fourniture de ces informations entrainera l’impossibilité pour QIMA Life Sciences de vous répondre.
Expt. 1 Introduction to in vitro pharmacology and physiological salt solutions
This document provides an overview of in-vitro pharmacology experiments using isolated tissues and physiological salt solutions (PSS). It defines pharmacology and drugs, describes the aims of experimental pharmacology as finding therapeutic agents, studying toxicity and mechanisms of action. It also discusses types of experiments, equipment like organ baths and levers for recording tissue responses, and PSS compositions and roles. PSS are artificial solutions that maintain isolated tissues by resembling extracellular fluid composition. Selection of the appropriate PSS depends on the tissue being studied. Read less

More Related Content
- 1. Experiment No. 1 Introduction to in-vitro pharmacology and physiological salt solutions (PSS) Mr. Vishal Balakrushna Jadhav Assistant Professor (Pharmacology) GES’s Sir Dr. M. S. Gosavi COPER, Nashik-5 1
- 2. Overview of Discussion Definitions of pharmacology & drug Aims of experimental pharmacology Pre-clinical pharmacology Clinical pharmacology Types of experiments in pharmacology Assembly for isolated organ/ tissue related experiments Recording (writing) levers Physiological salt solution (PSS) Introduction Examples Composition Role of ingredients Precautions in preparation of PSS Selection of PSS 2
- 3. Definitions of pharmacology & drug Pharmacology The science which deals with the study of drugs. The word ‘pharmacology’ is derived from the Greek words- Pharmakon (a drug or poison) and logos (discourse/ study/ science). It broadly covers the information about the history, source, physicochemical properties, and physiological actions, mechanism of action, absorption, distribution, metabolism, excretion and therapeutic uses of drugs→ PK and PD profile of drug. Drugs The chemical substances used for the purpose of diagnosis, prevention, relief or cure of a disease in man or animals. The word drug derived from the French word ‘drogue’ meaning herb. 3
- 4. Aims of experimental pharmacology The main aims of experimental pharmacology are to- (1) find out therapeutic agents suitable for human use, (2) study the toxicity of a drug, and (3) study the mechanism and site of action of drugs. Experimental pharmacology involves the discovery of new drugs or to study the actions of existing drugs. Divisions of experimental pharmacology- i) Preclinical pharmacology which involves the identification and optimization of novel chemical lead structures and testing on animals and animal tissues or organs for their biological action, and ii) Clinical pharmacology where testing of drugs is done on human volunteers and patients for assessing the pharmacokinetic, safety and efficacy in humans. 4
- 5. Definitions of pharmacology & drug Pharmacology The science which deals with the study of drugs. The word ‘pharmacology’ is derived from the Greek words- Pharmakon (a drug or poison) and logos (discourse/ study/ science). It broadly covers the information about the history, source, physicochemical properties, and physiological actions, mechanism of action, absorption, distribution, metabolism, excretion and therapeutic uses of drugs→ PK and PD profile of drug. Drugs The chemical substances used for the purpose of diagnosis, prevention, relief or cure of a disease in man or animals. The word drug derived from the French word ‘drogue’ meaning herb. 5
- 6. Aims of experimental pharmacology The main aims of experimental pharmacology are to- (1) find out therapeutic agents suitable for human use, (2) study the toxicity of a drug, and (3) study the mechanism and site of action of drugs. Experimental pharmacology involves the discovery of new drugs or to study the actions of existing drugs. Divisions of experimental pharmacology- i) Preclinical pharmacology which involves the identification and optimization of novel chemical lead structures and testing on animals and animal tissues or organs for their biological action, and ii) Clinical pharmacology where testing of drugs is done on human volunteers and patients for assessing the pharmacokinetic, safety and efficacy in humans. 6
- 7. Types of experiments in pharmacology 7
- 8. Assembly for isolated organ/ tissue related experiments 8 Figure- Student organ bath and Sherrington’s drum revolving machine
- 9. Student organ bath The tissue bath used to put the animal tissue for studying the drug actions is called as student organ bath. This was first designed by Rudolph Magnus in 1904. The organ bath essentially consists of- a) an outer jacket (water bath) made up of steel, glass or perspex, b) the inner organ or tissue bath made up of glass with a capacity varying from 10 to 50 ml, c) thermostatically controlled heating rod, d) stirrer to keep the water in the jacket at uniform temperature, e) oxygen or delivery glass tube which also serves as tissue holder, and f) glass coil, one end of which is connected to the lower end of the organ bath and the other to the container having the physiological salt solution. The glass coil is usually of double the capacity of inner organ bath to ensure warming up of the solution before it enters the organ bath. The student organ bath having two units of inner organ tissue bath is called double unit organ bath. 9
- 10. Sherrington’s drum revolving machine It is used to move the kymograph at a fixed speed. The drum (with 152 mm of diameter) on which the kymograph is pasted is fixed on the shaft of Sherrington’s drum revolving machine and this drum revolves at a fixed speed around the shaft. The shaft has a groove in which movable metal block is present. This movable metal block is used to elevate or lower the position of drum. It is elevated or lowered by rotating the screw located at the top of shaft. The base of Sherrington’s drum revolving machine has three basal screws meant for horizontal leveling of machine. The gears in the basal part are for controlling the speed of rotating drum. The clutch on the backside of machine is to start or stop the rotation of the drum during recording of responses. Generally, rotation speed of this machine ranges from 0.12 mm/second to 500 mm/second or more. The strikes are used to provide electrical stimuli to the isolate tissue at a particular frequency. 10
- 11. Recording (writing) levers They are used to record the contractions or relaxations of the isolated tissue preparations. The recording is done on smoked papers fixed on circular cylinders (of different diameters) and run at different speed using electrical recording drums. The speed of the drum is adjusted depending upon the nature of experiment. The writing levers are light, rigid and are generally made up of wood (straw), light aluminium or stainless steel. The levers are of two types- (i) Isotonic type or type-I levers, which records the change in length due to contraction when the tension or applied load remains constant. Examples of isotonic levers are simple lever, frontal writing lever etc., and (ii) Isometric type or type-II levers, which records increase in the tension of the tissue due to contraction when the length of the tissue is kept constant. These are used in special circumstances such as recording muscle twitches produced by electrical stimulation. For recording such observations isometric strain-gauge transducer may be preferred. Examples of isometric levers are Starling’s heart lever, Brodie’s universal lever, gimble lever etc. 11
- 12. 12 Fig. Different types of recording levers
- 13. (a) Simple lever (side way writing) It is used to record isotonic contractions in the isolated tissue. It is the simplest type of lever made up of wood, stainless steel or aluminium. A celluloid writing tip (stylus) is attached at the end of the longer arm. The contractions are recorded as curved lines. Uncontrolled friction between stylus and kymograph is a major disadvantage of simple lever. (b) Frontal writing lever (writes frontally) It is used to record isotonic contractions in the isolated tissue. This lever is designed in such a way that the writing point rotates freely about its axle. This helps in reducing the tension between the smoked paper/ kymograph and the recording tip. The contractions are recorded as straight line. (c) Starling’s heart lever It is used to record isometric contractions in the isolated tissue. This lever is used to record rapid and multiple contractions in the isolated tissues like frog’s isolated and perfused heart. In this, the horizontal arm of the lever is suspended to a rigid point with a spring. The difference between this and other isotonic levers is that the fulcrum lies at one end beyond the point of attachment. 13
- 14. (d) Brodie’s universal lever It is a general utility lever. (e) Gimbal lever It is used to record isometric contractions in the isolated tissue. The friction between the writing end and the kymograph is minimum because the pressure on the stylus on the kymograph is depends on the gravity. (f) Paton’s auxotonic lever It is designed in such a way that the load on the tissue goes on increasing as the tissue contacts. In short, this type of lever is used to record change in length of tissue due to contraction with respect to change in applied load or tension or force of contraction. 14
- 15. Physiological salt solution (PSS)- Introduction The suitable solution providing the ionic requirements and nutritional supply to the isolated tissue/ organ under study is called as physiological salt solution (PSS). It provides an artificial media resembling the inorganic composition of blood plasma together with a buffer mechanism to maintain the optimum pH and glucose to facilitate tissue metabolism. It contain a mixture of cations, anions and glucose in distilled water. It is also called as PSS or Ringer solution. A solution of a salt or salts that is essentially isotonic with tissue fluids or blood; (especially: an approximately 0.9% solution of sodium chloride) → Therefore also called as normal saline, OR brine (salt water), OR saline solution, OR normal salt solution, OR physiological saline solution, OR physiological salt solution. It is an artificially prepared solution used to maintain tissues in viable state. PSS can be used to keep isolated tissue alive under experimental condition. PSS is very important to maintain tissue outside the animal body and fulfills their internal environment of ions and nutrition. 15
- 16. Physiological salt solution (PSS)- Examples 16
- 17. Ringer, frog ringer and De-Jalon solution do not contain phosphate (PO42-) ions. The composition of De-Jalon solution is same as that of Ringer-Locke solution except that it contains 1/4th the amount of calcium chloride (CaCl2) and ½ the amount of glucose. McEwen solution in addition contains sucrose. Frog ringer solution may also be prepared by adding 400 ml distilled water to one litre of Ringer-Locke solution (single glucose). Ringer solution is aerated with oxygen or air and used for mammalian isolated heart and other tissue, frog ringer is used frog heart and other isolated tissue preparations. Tyrode solution is aerated with air, oxygen or 5 % CO2 in oxygen and used for mammalian smooth muscles. Krebs and McEwen solutions are aerated with 5 % CO2 in oxygen and used for mammalian isolated organ especially for nerve responses. 17
- 18. Physiological salt solution (PSS)- Composition 18 The main components of PSS are Sodium Na+ Potassium K+ Calcium Ca2+ Chloride Cl- Glucose Magnesium Mg2+ Distilled Water (D. W.) De-ionized Water (D. W.) Doubled distilled Water (D. W.) Bicarbonates HCO3- Phosphate Buffer
- 19. Physiological salt solution (PSS)- Role of Ingredients 19 Sodium Na+ Potassium K+ Calcium Ca2+ Magnesium Mg2+
- 20. 20 Bicarbonates HCO3- Phosphate Buffer (KH2PO4/ NaH2PO4) Glucose Water Energy source, provide nutrition Vehicle/ Solvent
- 21. Physiological salt solution (PSS)- Precautions in preparation of PSS 21 Maintain PH = 7.3- 7.4 Hygroscopic Cacl2 & Mgcl2 salts to be added from stock solution Replacement of Mgcl2 with MgSO4 do not interfere with tissue activity Risk of bacterial growth due to glucose→ if stored for longer duration. CaCl2 added last → to prevent precipitate (PPT) formation or chelation of bicarbonate. (PPT makes solution turbid It interferes with internal property of solution & may reduce visibility of tissue).
- 22. 22 Add glucose & calcium at the time of Expt. if needed and required to store for 24 hrs. PSS should be stored for 24 hours in refrigerator Always prepare fresh solution Tissue aeration with the mixture of 95% O2+ 5% CO2 (Carbogen) O2 is Important for survival of tissue. Pure O2 interact with bicarbonate (HCO32-) in PSS causing CO2 loss to produce alkaline solution. Error should be less than 1%
- 23. Physiological salt solution (PSS)- Selection of PSS 23 Tyrode solution → For non-innervated muscles Krebs solution → For innervated muscles Frog ringer solution → For amphibian tissues Ringer Locke solution → For isolated amphibian heart Tyrode solution → Smooth muscles Ringer Locke solution → For isolated amphibian heart Krebs, De Jalon’s and Mc-Ewen solution → For avian skeletal muscles and other innervated muscles De Jalon’s solution → For isolated tissues of rabbit
- 24. 24 Thank you!
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Markossian S, Grossman A, Arkin M, et al., editors. Assay Guidance Manual [Internet]. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2004-.

Assay Guidance Manual [Internet].
In vitro and in vivo assessment of adme and pk properties during lead selection and lead optimization – guidelines, benchmarks and rules of thumb.
Thomas D.Y. Chung , David B. Terry , and Layton H. Smith .
Affiliations
Published September 9, 2015 .
Assessment of the pharmacological properties of small molecule chemical compounds is critical to the initial selection or identification of a chemical lead, and during the further lead optimization to elucidate the Structure-Activity Relationships (SAR) and Structure Property Relationships (SPR), and ultimately to select the compound(s) that will enter Investigational New Drug (IND)-enabling studies. While extensive discussion of how Absorption, Distribution, Metabolism, and Excretion (ADME) of compounds affects their ultimate pharmacokinetics (PK) is beyond the scope of this chapter, herein, we provide guidelines for ADME and PK assessments, benchmarks and practical “rules of thumb” for selecting compounds with sufficient PK to be viable efficacious drugs.
- Flow Chart of a Two-tier Approach for In Vitro and In Vivo Analysis

As well-reviewed in the Assay Guidance Manual (AGM) chapter on Early Drug Discovery and Development Guidelines, there is an evolving paradigm for drug discovery and early development with the academic and non-profit enterprises focused on delivering innovative, novel, new chemical entities (NCE) through collaboration, partnering and licensing optimized leads for final clinical development to pharmaceutical companies. That chapter outlined the overall process, critical steps and key decision points at each step. Each of these steps and associated technologies, protocols, techniques, and case examples are covered elsewhere in the AGM. Ultimately, an exemplary compound(s) emerges from systematic elucidation of the Structure Activity Relationship (SAR) through examining the potency, specificity and selectivity of analogs around a chemical scaffold. This comprises identification of a chemical lead, where the most potent, specific and selective compound(s) are chosen.
Assessments of the pharmacological properties of Absorption, Distribution, Metabolism, and Excretion (ADME) of a candidate chemical lead(s) are critical to their initial selection, and establishes benchmarks against which compounds synthesized during lead optimization can be evaluated. Further improvements in ADME properties during lead optimization are sought, while preserving the potency and selectivity of the chemical lead(s), though sometimes more efficacious compounds have lower in vitro potencies, but better ADME properties.
These activities often reside in an exploratory pharmacology group that provides in vitro and in vivo pharmacologic and physicochemical property analysis of biologically active small molecules in support of small molecule probe/drug discovery projects. Early pharmacological assessment has been adopted within the pharmaceutical industry as a critical feature of a robust drug/probe discovery process. This is because the development and optimization of useful molecules is a multi-parameter process. Simply designing new analogs and developing a SAR for increased potency against the biological target is inadequate for the development of small molecule probes or drugs suitable for cellular, tissue, or whole animal disease model(s). The assessment and optimization of Structure-Pharmacologic/Property-Relationships (SPR) is a further critical step for efficacy evaluation. In addition to assessing compound characteristics such as solubility, protein binding, and serum stability, the data allows the chemistry team to prioritize different structural classes and rank order them not only based on potency but also in relation to potential downstream absorption or metabolism liabilities. An excellent additional overview of pharmacokinetics (PK) can be found on the online version of the Merck Manual ( http://www.merckmanuals.com/professional/clinical-pharmacology/pharmacokinetics/overview-of-pharmacokinetics )
Prior to actual dosing in animals, a number of relatively rapid and cost effective in vitro assays can serve as surrogates and indicators of the ADME fate of compounds in vivo . Improvements in ADME properties of compounds translate to their improved PK properties. Simply stated, if a compound is rapidly absorbed, well distributed, minimally metabolically degraded and not rapidly eliminated, while not being toxic, then it more likely will rapidly achieve peak levels in the blood, maintain the desired levels (n-fold above the IC 50 ) for a longer duration, before falling to low trough levels, and ultimately being cleared by the body.
In the sections below, we describe the basic component and provide high level protocols for a two-tiered approach for these key in vitro ADME assays (see Flow Chart). We provide an example of an ADME table, and benchmarks for a series of probe compounds developed through the NIH Molecular Libraries Program (MLP) by our group when we were part of the Molecular Libraries Probe Production Centers Network (MLPCN). We then summarize a two-tiered approach to doing pharmacokinetic studies (see Flow Chart). First an abbreviated “rapid” assessment for compound exposure (R.A.C.E.) developed by us (LHS), then the more typical “comprehensive” pharmacokinetic analysis used during late lead optimization toward candidate selection for final preclinical IND.
Ultimately, the in vivo efficacy of an optimized lead will be better served by having good pharmacological properties, so that the compound administered at a given dose actually achieves the required concentration, for sufficient duration in the target tissue to achieve the desired biological effect, while minimizing any undesired off target effects. Improvement in these ADME properties is sought prior to actual dosing in animals to assess PK, and certainly for larger compound efficacy studies, since animals are expensive and the ethics of sacrificing animals in poorly designed studies uninformed by pharmacological guidance are indefensible.
- In Vitro Analysis - Low Compound Requirements and Relative Moderate Capacity
Lipophilicity
Pharmacologic question addressed: “ Will my parent compound be stored in lipid compartments or how well will my parent compound bind to a target protein? ”
Lipophilicity is an important physicochemical property of a potential drug. It plays a role in solubility, absorption, membrane penetration, plasma protein binding, distribution, CNS penetration and partitioning into other tissues or organs such as the liver and has an impact on the routes of clearance. It is important in ligand recognition, not only to the target protein but also CYP450 interactions, HERG binding, and PXR mediated enzyme induction.
Lipophilicity is typically measured as the neutral (non-ionized) compound distribution between non-aqueous (octanol) and aqueous (water) phase and the result is expressed as a 10-base logarithm of the concentration ratios between these phases (partition coefficient), log P.
Another common measure for lipophilicity is the distribution coefficient, log D, which takes into account the compound’s ionized and non-ionized forms, and therefore the measurement is done at different pH values. Typically the most interesting is pH 7.4, since the majority of known drugs contain ionizable groups and are likely to be charged at physiological pH.
Assay Design:
- Test articles are assayed in triplicate
- One concentration of test article (typically 10 μM)
- n-Octanol is the partition solvent
- Ratio of buffer: Octanol is 1:1 (other ratios available)
- Positive control : Testosterone (high log D 7.4 value)
- Negative control : Tolbutamide (low log D 7.4 value)
Analysis : LC/MS/MS measurement of parent compound
Report : Log D 7.4 value
Quantity of test article required : 1.0 - 2.0 mg
Summary of Assay :
Lipophilicity of compounds is assessed using the golden standard “shake-flask” method. The compound is dissolved in a solution with equal amounts of octanol and water, shaken for 3 hours, and then measured for the amount of compound in each phase. Log D values are calculated by the log ([compound] octanol / [compound] buffer ).
Pharmacologic question addressed: “ What is the bioavailability of my compound? ”
Aqueous solubility, another common physicochemical parameter for drug discovery compounds, is an important analysis as it reflects the bioavailability of the compound. The ability of a compound to dissolve in a solvent to give a homogenous system is one of the important parameters to achieve a desired concentration of drug in systemic circulation for the desired (anticipated) pharmacological response. Formulation and routes of administration, especially oral dosing, are challenging for poorly soluble drugs, as it limits the absorption of compound from the gastrointestinal tract. Also, poor solubility will affect other AMDE/DMPK analyses, if some fraction of the compound precipitates and is unavailable (e.g. in assays for metabolite stability and various CYP identification/inhibition/induction assays). Also, since the majority of known drugs contain ionizable groups, the aqueous solubility is assessed over a range of pH values.
Assay Design :
- Test articles are assayed in duplicate
- One concentration of test article (typically 1 μM)
- Phosphate buffered solution (other buffers available)
- Three point pH range (5.0, 6.2, 7.4)
- Positive control : Diclofenac (high solubility)
- Negative control : Dipyridamole (low solubility)
- Background control : DMSO only
Analysis : UV spectrophotometry measurement of parent compound
Report : Amount of compound dissolved (μM)
The compound is dissolved in buffer solutions at the indicated pH values. The compound is allowed to reach thermodynamic equilibrium by incubating for 18 hours. Compound UV absorption is compared to fully saturated solution in 1-propanol.
Hepatic Microsome Stability
Pharmacologic question addressed: “ How long will my parent compound remain circulating in plasma within the body? ”
The assay uses subcellular fractions of liver, microsomes, to investigate the metabolic fate of compounds. Liver microsomes consist mainly of endoplasmatic reticulum and contain many drug-metabolizing enzymes, including cytochrome P450s (CYPs), flavin monooxygenases, carboxylesterases, and epoxide hydrolase ( 1 ). Liver microsomes are available commercially (example, Xenotech, LifeTechnologies and DB Biosciences) as frozen preparations that are usually prepared in bulk with pooled livers from sacrificed mice, rat or human cadavers. As a result, hepatic microsomal metabolic activity can vary significantly from batch to batch. Therefore, in critical studies, it is recommended that planning to obtain the same lot of microsomes be considered in the experimental plans. If lots of microsomes do run out, a few bridging comparisons to establish comparable values for microsomal stability of reference compounds, should be done.
- Human liver microsomes (or other species as needed) (0.5 mg/mL)
- One concentration of test article (typically 10 μM)*
- Two time points t = 0 and t = 60 min*
- Positive control : Substrates with known activity
- Negative control : NADPH deficient
Analysis : LC/MS/MS measurement of parent compound at specific time points
Report : % metabolism of the test article (single time point); also, intrinsic clearance and half-life (multiple time points)*
Summary of Assay:
Metabolic stability of compounds are assessed at a single concentration (typically 10 μM) at t = 0 and at t = 60 min. Stability of compounds are tested in human (other species available) liver microsomes. Compounds are tested in triplicate with or without NADP wells as a negative control for P450 metabolism. Each assay will include a substrate with known activity (such as the CYP3A4 substrate testosterone) as a positive control ( 2 ).
*Note: the number of concentrations/time points in the assay can be expanded for drug development SAR efforts.
Plasma Stability
Pharmacologic question addressed: “ Is my compound degraded in plasma? ”
In addition to hepatic metabolism, compounds are also subjected to degradation/modification by enzymes in plasma, particularly hydrolysis and esterases. Thus, the stability of test compounds in plasma is an important parameter, which not only affects in vivo results, but also the bioanalytical assay strategy and design. Investigation of plasma stability should be performed early in the discovery process in order to assess potential degradation and/or protein binding issues.
- Two concentrations (10 μM and 100 μM or if known C max and 10x C max )
- Two time points t = 0 and t = 180 min
- Positive control : Procaine (50 μM)
- Negative control : Procainamide (50 μM)
Analysis : LC/MS/MS detection of the remaining test article
Report : % parent compound remaining
A solution of test compound in plasma is prepared and incubated for a predetermined time period. Aliquots are removed at pre-defined time points and analyzed by LC/MS/MS. The peak area for the parent compound is compared to the time zero sample in order to assess the amount of compound still available.
Plasma Protein Binding
Pharmacologic question addressed: “ What percent of the compound plasma protein is bound, to which component (sub-fraction), and what is the free fraction available to cover the target? ”
The binding of test compounds to plasma proteins is an important factor affecting drug efficacy, metabolism and pharmacokinetic properties. In many cases, drug efficacy is determined by the concentration of free drug (unbound), rather than the total concentration in plasma. If the drug is highly bound to plasma proteins, the amount of drug available to reach the target is reduced. Subsequently, the efficacy of that compound may be significantly reduced. Therefore, information on the free drug fraction is essential for drug development and may be helpful in correlating with in vivo efficacy.
Rapid equilibrium dialysis (RED) is an accurate and reliable method for determining the degree to which a compound binds to plasma proteins. Plasma spiked with test compound is added to the center chamber of a commercial plate based RED device. Blank, isotonic sodium phosphate buffer is added to the peripheral chamber of the RED device and the plate is incubated at 37°C for 4 hours. Equilibrium of free compound is achieved by the diffusion of the unbound compound across the dialysis membrane. Several manufacturers provide RED devices (Thermo Scientific). Aliquots of the buffer and the plasma are taken at pre-determined time points and the concentration of free and bound test compound is determined by LC/MS/MS analysis.
- Test articles are mixed with human plasma (other species available)
- One concentration of test article (10 μM, different concentrations available)
- One time point ( t = 4 hours at 37°C)
- Positive control : Propranolol (high binding) and Metoprolol (low binding)
- Negative control : No plasma (PBS only)
Analysis : LC/MS/MS detection of the test compound in plasma and in buffer
Report : % compound bound
Human or specific species of interest plasma in the sample chamber are spiked with test compounds at 100x dilution of stock solution (typically 10 mM in DMSO). The chamber is sealed, and the compound is dialyzed against PBS, pH 7.4 at 37°C for 4 hours. Aliquots from each chamber (plasma and PBS) are collected and the concentrations of compound in each sample are determined by LC/MS/MS. Adjustments are made for non-specific binding.
Screening Cytotoxicity / Hepatotoxicity Test
Pharmacologic question addressed: “ Is my compound too toxic to be therapeutic? ”
Cytotoxicity is a well-established and easily accessible endpoint to gather early information about the general / acute toxic potential of a test article. The in vitro cytotoxicity test with primary hepatocytes is used to identify the cytotoxic potential of a test substance. The relative cell viability upon incubation with test article compared to the solvent control is determined (single point).
The ATP-lite 1step Cytotoxicity Assay (PerkinElmer) is a single reagent addition, homogeneous, luminescence ATP detection assay that measures the number of live cells in culture wells.
- Primary hepatocytes (other cells available)
- 12-dose concentration response curve ( CRC ) of the test article (100x IC 50 or 50 µM maximum concentration)
- Two replicates of CRC
- One incubation time 24 hours
- Positive control : Compounds with known toxicity
- Negative control : Compound with known non-toxicity
- Background control : Vehicle only
Analysis : Luminescence is measured from 550 - 620 nm.
Report : IC 50
Hepatocyte cells are incubated for 24 hours with known toxic and non-toxic compounds at a range of different concentrations. At the end of the incubation period the cells are loaded with the ATP-lite TM 1step ATP monitoring reagent and scanned using an automated plate reader with luminescence detection (Tecan Infinite M200 reader) to determine the number of active cells.
CYP450 Inhibition Profiling
This assay extends the findings of the microsomal stability assay. Pharmacologic question addressed: “ Does my compound inhibit a key oxidative metabolic enzyme that would lead to subsequent drug-drug interactions? ”
Cytochrome P450s (CYPs) are a superfamily of heme-containing enzymes that mediate the inactivation and metabolism of many drugs as well as endogenous substances. Compounds that inhibit P450s may cause the toxic accumulation of other substrates. CYP inhibition profiling examines the effects of a test compound on the metabolism of other known enzyme substrates of the five primary drug human metabolizing CYP: 1A2, 2B6, 2C9, 2D6, 3A4. The levels of the CYP isoform marker substrate and metabolites are measured in the presence and absence of a test compound by LC/MS/MS.
- Five CYP isoenzymes: 1A2, 2B6, 2C9, 2D6, 3A4
- Test articles are run in triplicate
- One concentration of human liver microsomes (0.5 mg/mL)
- One concentration of test item (10 µM)
- One time point 0.5 hours
- Positive control : CYP marker reaction ( Table 1 )
- Negative control : NADPH deficient reaction control
Inhibition profiling for the five primary drug human metabolizing cytochrome P450s.
Analysis : LC/MS/MS detection (appearance of metabolite)
Report : Data are expressed as % inhibition of selected metabolites formation for each CYP450 enzyme (1A2, 2B6, 2C9, 2D6, 3A4).
(See FDA guideline for CYP substrates:
http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm )
In an assay similar to the metabolic stability assay, liver microsomes are used to determine the CYP450 inhibition profile of test compounds by measuring the % metabolism of a known substrate. Microsomes (and NADPH regenerating system) are dispensed into a 96-well plate containing a substrate and test compound (10 µM), and the reaction is allowed to proceed for 0.5 hours at 37°C with shaking. The reaction is quenched by the addition of MeOH, centrifuged and the amount of product is measured by LC/MS/MS. Each plate will contain a known inhibitor of each CYP450 profiled as positive control and NADP-/- negative controls.
Permeability
Pharmacologic question addressed: “ How well is my drug absorbed in the gastrointestinal tract? ”
Evaluating compound permeability through a cell monolayer is a good indication of intestinal permeability and oral bioavailability. The Parallel Artificial Membrane Permeability Assay (PAMPA) provides a high throughput, non-cell based method for predicting passive, transcellular intestinal absorption, the process by which the majority of small molecule drugs enter circulation. In the PAMPA method, an artificial membrane immobilized on a filter is placed between a donor and acceptor compartment. The compound is introduced in the donor compartment. Following the permeation period, the amount of compound in the donor and acceptor compartments are quantified using scanning UV spectrophotometry.
The gastrointestinal tract (GT) has a pH range from pH 1 – 8. The pH of the blood is constant at pH 7.4; therefore it is possible for a pH gradient to exist between the GT and the plasma that can affect the transport of ionizable molecules. In an effort to mimic this pH gradient in vitro , alternative assays with pH 7.4 for the acceptor compartment and pH values 5.0, 6.2, and 7.4 in the donor compartment are used.
PAMPA is a well-established and predictive assay that models the absorption of drugs in the gut. However, PAMPA is an artificial system that may provide inaccurate and potentially misleading results. Despite these limitations, PAMPA can be a useful tool to prioritize lead compounds in early stages of development. The colon carcinoma (Caco-2) cell permeability assay is the industry standard for in vitro prediction of intestinal absorption of drugs, but it too has limitations. Caco-2 cells require extensive culturing (>20 days), and often fail to form the cohesive monolayer necessary for uniform transport of compounds across the cell layer. The assay requires a significant amount of compound to perform the assay (typically ~20 mg). Together, the limitations of time and compound consumption decrease the value of the results obtained by Caco-2 at the early stages of drug discovery. One variant of PAMPA is the Blood Brain Barrier (BBB) PAMPA in where the artificial monolayer contains brain specific membrane components, such as sphingolipids.
- One concentration (25 µM)
- One time point (18 hours)
- One pH (7.4) or three point pH range (5.0, 6.2, 7.4) for acceptor compartment
- Single polar membrane lipid (phosphatidylcholine in dodecane)
- Multiscreen PVDF membrane (0.45 µm)
- Positive control : Verapamil (high permeability)
- Negative control : Theophylline (low permeability)
Analysis : The concentration of the compound remaining in the donor well, diffused through the membrane and into the acceptor well, and reference compounds are measured by UV spectrophotometry
Report : Bin the results as high, medium, or low predicted absorption and report direct permeability units (10-6 cm/s)
Quantity of test article required : 5.0 - 7.0 mg
A lipid bilayer is established on a membrane filter and a test compound solution is added to the top of the membrane-lipid interface. The ability of compounds to passively diffuse through the lipid treated membrane is an indication of the overall compound permeability. This approach is helpful in compound profiling and supporting the relative rank ordering of compounds.
- Example of In Vitro ADME Profiling Assays
Table 2 below shows an example tabulation of the ADME properties assessed and evaluated as a requirement for nomination of a chemical probe in the Molecular Libraries Program (MLP) for probes ML301, ML314 and a few key related analogs, as described in the NCBI MLP probe report: http://www.ncbi.nlm.nih.gov/books/NBK184496/ that describes a novel small molecule agonist for the Neurotensin 1 Receptor (NTR1).
Summary of in vitro ADME/T properties of NTR1 agonists ML301 (& analogs) and ML314.
ADME profile of ML301 (Probe 1) and related compounds: ML301, its intriguing naphthyl analog (MLS-0446079), and the prior art, pyrazole, were evaluated in a detailed in vitro ADME screen as shown in Table 2 . Despite its structural similarity (imidazole vs. pyrazole) to the prior art compound, ML301 exhibited substantial advantages in this testing, especially with regard to plasma and microsomal stability. These three compounds all exhibited good solubility due to the presence of the carboxylic acid moiety.
The PAMPA assay is used as an in vitro model of passive, transcellular permeability. The compounds exhibited good overall permeability, inversely related to the pH of the donor compartment. Because these NTR1 agonists are envisioned as predecessors of psychoactive drugs, a preliminary assessment of their potential to cross the BBB was performed. When incubated with an artificial membrane that models the BBB, much lower permeability was observed. These observations are also consistent with the carboxylic acid function in the compounds, and may present an opportunity for future enhancements.
Plasma protein binding is a measure of a drug's efficiency of binding proteins within blood plasma. The less bound a drug is, the more efficiently it can traverse cell membranes or diffuse. Drugs that are highly bound to plasma proteins are confined to the vascular space, thereby having a relatively low volume of distribution. In contrast, drugs that remain largely unbound in plasma are generally available for distribution to other organs and tissues. The imidazole scaffold compounds (ML301 and its MLS-0446079) exhibited substantial protein binding, but significantly lower than that of the prior art, pyrazole.
The stability of small molecules and peptides in plasma may strongly influence in vivo efficacy. Drug candidates are susceptible to enzymatic processes, such as those mediated by proteases or esterases in plasma. They may also undergo intramolecular rearrangement or bind irreversibly (covalently) to proteins. ML301 showed excellent stability in plasma, significantly better than that of either analog.
The microsomal stability assay is commonly used to rank compounds according to their metabolic stability, which influences how long the candidate may remain intact while circulating in plasma. ML301 showed excellent stability in human and modest stability in mouse liver homogenates, which was much better than that observed for the prior art analog, MLS-0437103. None of the compounds showed toxicity (>50 µM) toward human hepatocytes.
ADME profile of ML314 (Probe 2): As described above for ML301, in vitro ADME screening was also conducted for ML314. Consistent with its aqueous solubility data, ML314 exhibited high permeability in the PAMPA assay with increasing pH of the donor compartment. When incubated with an artificial membrane that models the BBB, ML314 was found to be highly permeable. ML314 was highly bound to plasma protein and exhibited very high plasma stability. ML314 was metabolized rapidly when incubated in vitro with human and mouse liver microsomes. This result is not completely surprising because of the presence of several unsubstituted aryl and alkyl positions and Ar-OMe ethers, which are prone to oxidation, hydrolysis, conjugation and other metabolic reactions. ML314 showed a >15-fold window for toxicity (LC 50 = 30 μM) towards human hepatocytes. Improving the metabolic stability and toxicity profile of ML314 represents a challenge as well as an avenue for further optimization studies in the future.
- In Vivo Analysis - High Compound Requirements and Low Capacity
Rapid Assessment of Compound Exposure (R.A.C.E.)
This experiment is a rapid and efficient compressed in vivo PK screening method to determine the pharmacokinetic attributes of novel chemical probes ( 3 ). A small cohort of animals (4 mice or 2 rats/experiment), is administered compound orally ( p.o .) or by intraperitoneal injection ( i.p .) at a single dose (5 - 50 mg/kg). Blood samples are collected at 20 and 120 minutes. The plasma samples are analyzed with a mini-standard curve ( Figure 1 ). This experiment provides a snapshot of compound exposure sufficient to estimate total compound exposure as the area under the curve (AUC (20-120 min) ), providing a rank order of estimated AUC values to prioritize compounds for further investigation.
Example of data from a previously performed RACE study. The purpose of this study was to select which compound from a series exhibited the highest estimated exposure. (A) Representative exposure data from two compounds in a series obtained by sequential (more...)
Another utilization for R.A.C.E. is to evaluate varying formulation excipients for improving solubility and absorption. Generally, formulation excipients approved by the FDA are available for this assay. Please refer to reference ( 4 ) for FDA approved excipients and amounts.
- Test articles are formulated for p.o. or i.p. dosing
- One dose of test article (5 – 50 mg/kg)
- Two time points ( t = 20 min and 120 min)
Analysis : LC/MS/MS detection of the test compound(s) in plasma samples
Report : Estimated AUC (20-120 min) and a rank order of compound exposure
Quantity of test article required : 5.0 - 50.0 mg
Comprehensive Pharmacokinetic Analysis
Compounds that show promising PK profiles or that are further developed, can be subjected to a comprehensive pharmacokinetic analysis and metabolite identification study. An example set of graphs representing typical time course drug plasma concentrations following oral dosing can be found in Wikipedia http://en.wikipedia.org/wiki/Pharmacokinetics and are reproduced to illustrate the key related parameters: time to reach (t max ) maximal concentration (C max ) of compound, time to reduce concentration by half of the initial value (t 1/2 ), dosing interval (τ), and area-under-curve (AUC) or total compound exposure ( Figure 2 ).
Time course of drug plasma concentrations over 96 hours following oral administrations every 24 hours (τ). Absorption half-life is 1 hour and elimination half-life is 12 hours. Note that in steady state and in linear pharmacokinetics AUC τ (more...)
A minimum of six 300 gram rats are required, per compound tested, for appropriate sampling. For each drug, the compound will be formulated in suitable vehicle to typically 1.0 mg/mL (final concentration) and administered to 3 rats at 1 mg/kg i.v . into a femoral vein catheter. The same substance is administered to an additional 3 rats at 2 mg/kg by oral gavage ( direct dosing of compound into the stomach through a feeding tube ). Blood is drawn (0.25 mL) via the femoral artery catheter at 5, 15, and 30 minutes and at 1, 2, 4, 6, 8 and 24 hours post dose for a total maximum volume of 2.25 mL. Plasma samples are analyzed by LC/MS/MS. A comprehensive PK analysis requires 6-12 animals and blood collections at 8 time points.
- Single test article is formulated for both p.o . and i.v . dosing
- I.v and p.o. doses of test article (e.g., 1.0 mg/kg and 2.0 mg/kg)
- Nine time points (5, 15, and 30 min and at 1, 2, 4, 6, 8 and 24 hours).
Analysis : LC/MS/MS detection of the test compound(s) in individual plasma samples
Report : Time course of plasma drug concentration versus time, PK parameters (for example, AUC, t 1/2 , oral bioavailability) and metabolite identification
Quantity of test article required : 10 - 100 mg
- Suggested Equipment and Resources
Automated workstation: A flexible versatile high-throughput/high-capacity workstation system for automated liquid handling is a useful work horse of the pharmacology lab. The system should be capable of processing compound solutions for analysis and executing the various in vitro profiling assays. A variable spanning 8 channel pipette arm, a 96-well multichannel pipetting head, and plate gripper arm are useful for reformatting samples between vials, tubes, and 96-well plates. Specialized devices include vacuum filtration and magnetic separation modules, as well as integrated devices for heating, cooling, and shaking samples.
Multimodal Plate Reader: A multi-mode plate reader capable of UV/Vis, top/bottom fluorescence, and flash/glow luminescence read modes accessible to the plate gripper arm of a workstation is useful for unattended reading for a batch of samples.
Liquid Chromatography/Mass Spectrometric/Mass Spectrometry (LC/MS/MS): This technology is the workhorse of all ADME/T and PK analyses of samples from both the in vitro and in vivo assay. After extraction of biomatrix from small molecules, the small molecule sample and any related compounds (metabolized, hydrolyzed, or broken down compounds) are first separated through high-performance liquid chromatography (HPLC). The effluent from the HLPC are monitored in real-time for ultraviolet absorbance at 254 and 280 nm, and absorbing samples are diverted into an on-line electrospray unit to introduce samples into the mass spectrometer, followed by sequential quadrapole selection and detection of mass/charge (m/z) distributions of samples that are characteristic of parent and derived molecules. For example, we use a Shimadzu UPLC coupled with an automated AB Sciex API 4000 MS/MS system with QTRAP detection. This is a high performance hybrid triple quadrupole/linear ion trap system with excellent dynamic range and sensitivity. However, this is a very mature sector of the MS market and several vendors with comparable instruments exist.
Microplate Scintillation and Luminescence Counter: For studies with radiolabeled compounds, scintillation devices are still required. Currently a few manufacturers still make multi-wall, microtiter plate based scintillation counters (e.g. PerkinElmer TopCount NXT™), these devices can also plate luminescence readers.
Literature Cited
Additional references.
All Assay Guidance Manual content, except where otherwise noted, is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported license (CC BY-NC-SA 3.0), which permits copying, distribution, transmission, and adaptation of the work, provided the original work is properly cited and not used for commercial purposes. Any altered, transformed, or adapted form of the work may only be distributed under the same or similar license to this one.
- Cite this Page Chung TDY, Terry DB, Smith LH. In Vitro and In Vivo Assessment of ADME and PK Properties During Lead Selection and Lead Optimization – Guidelines, Benchmarks and Rules of Thumb. 2015 Sep 9. In: Markossian S, Grossman A, Arkin M, et al., editors. Assay Guidance Manual [Internet]. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2004-.
- PDF version of this page (290K)
- PDF version of this title (79M)
- Disable Glossary Links
In this Page
Assay guidance manual links.
- New in Assay Guidance Manual
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Pharmacokinetics in Drug Discovery: An Exposure-Centred Approach to Optimising and Predicting Drug Efficacy and Safety. [Handb Exp Pharmacol. 2016] Review Pharmacokinetics in Drug Discovery: An Exposure-Centred Approach to Optimising and Predicting Drug Efficacy and Safety. Reichel A, Lienau P. Handb Exp Pharmacol. 2016; 232:235-60.
- Pharmacokinetics and ADME characterizations of antibody-drug conjugates. [Methods Mol Biol. 2013] Pharmacokinetics and ADME characterizations of antibody-drug conjugates. Lin K, Tibbitts J, Shen BQ. Methods Mol Biol. 2013; 1045:117-31.
- Review Preclinical pharmacokinetics: an approach towards safer and efficacious drugs. [Curr Drug Metab. 2006] Review Preclinical pharmacokinetics: an approach towards safer and efficacious drugs. Singh SS. Curr Drug Metab. 2006 Feb; 7(2):165-82.
- Development and application of physiologically based pharmacokinetic-modeling tools to support drug discovery. [Chem Biodivers. 2005] Development and application of physiologically based pharmacokinetic-modeling tools to support drug discovery. Lüpfert C, Reichel A. Chem Biodivers. 2005 Nov; 2(11):1462-86.
- Review Strategy of utilizing in vitro and in vivo ADME tools for lead optimization and drug candidate selection. [Curr Top Med Chem. 2005] Review Strategy of utilizing in vitro and in vivo ADME tools for lead optimization and drug candidate selection. Balani SK, Miwa GT, Gan LS, Wu JT, Lee FW. Curr Top Med Chem. 2005; 5(11):1033-8.
Recent Activity
- In Vitro and In Vivo Assessment of ADME and PK Properties During Lead Selection ... In Vitro and In Vivo Assessment of ADME and PK Properties During Lead Selection and Lead Optimization – Guidelines, Benchmarks and Rules of Thumb - Assay Guidance Manual
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
A Brief Guide to Performing Pharmacological Studies In Vitro : Reflections from the EORTC-PAMM Course "Preclinical and Early-phase Clinical Pharmacology"
Affiliations.
- 1 Fondazione Pisana per la Scienza-ONLUS, Pisa, Italy.
- 2 Molecular Pharmacology Unit, Department of Applied Research and Technological Development, Fondazione IRCCS, Istituto Nazionale dei Tumori, Milan, Italy.
- 3 Amsterdam UMC, VU University of Amsterdam, Medical Oncology, Cancer Center Amsterdam, Amsterdam, the Netherlands.
- 4 Department of Biological, Chemical and Pharmaceutical Sciences and Technologies (STEBICEF), University of Palermo, Palermo, Italy.
- 5 Laboratory of Pharmacology, Department of Experimental Oncology, Institute for Oncology and Radiology of Serbia, Belgrade, Serbia [email protected].
- PMID: 31262863
- DOI: 10.21873/anticanres.13485
One aim of cell-based in vitro assays is to identify the best drug candidate to develop using the best tumor cell model. This is challenging in every anticancer drug discovery process. Briefly, we summarize the parameters to be taken into account when performing in vitro cell assays, in order to obtain reliable and reproducible results, which was fundamentally discussed by lecturers at the educational course on preclinical and early-phase clinical pharmacology studies, at the 40th Winter Meeting of the Pharmacology and Molecular Mechanisms Group of the European Organization for Research and Treatment of Cancer. Moreover, specific cellular sensitivity tests are described. In addition to monolayer in vitro cell models for the screening of new potential candidate drugs, three-dimensional tumor/cell tissue models are emerging as new pre-clinical tools that more closely reflect the in vivo microenvironment. Therefore, the use of different in vitro models for drug screening can enhance the predictability and reliability of pre-clinical drug-discovery phases and target validation.
Keywords: In vitro; anticancer drugs; cell-sensitivity assays; review; tumor cells.
Copyright© 2019, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Publication types
- Biological Assay
- Cell Culture Techniques
- Drug Evaluation, Preclinical*
- Pharmacology, Clinical / methods*

IMAGES
COMMENTS
in vitro, which refers to studies or experiments conducted on microorganisms and cells outside of their normal biological environment pharmacology , which is the study of the effects of drugs and pharmaceuticals on living organisms
EXPERIMENT NO: 1 INTRODUCTION TO IN-VITRO PHARMACOLOGY AND PHYSIOLOGICAL SALT SOLUTIONS. Aim: To study the Introduction about In-Vitro Pharmacology and Physiological salt solutions. Introduction: “In vitro” is a Latin word that means “within the glass” Therefore the studies which are done
In vitro testing has been used to characterize specific adsorption, distribution, metabolism, and excretion processes of drugs or general chemicals inside a living organism; for example, Caco-2 cell experiments can be performed to estimate the absorption of compounds through the lining of the gastrointestinal tract; [20] The partitioning of the ...
Aug 10, 2021 · This document provides an overview of in-vitro pharmacology experiments using isolated tissues and physiological salt solutions (PSS). It defines pharmacology and drugs, describes the aims of experimental pharmacology as finding therapeutic agents, studying toxicity and mechanisms of action.
Sep 9, 2015 · This experiment is a rapid and efficient compressed in vivo PK screening method to determine the pharmacokinetic attributes of novel chemical probes . A small cohort of animals (4 mice or 2 rats/experiment), is administered compound orally (p.o.) or by intraperitoneal injection (i.p.) at a single dose (5 - 50 mg/kg). Blood samples are collected ...
Aim: Types of Pre-clinical Experiments: In-Vivo, In-Vitro, Ex-Vivo, and More Pre-clinical experiments are essential in the early stages of drug development, providing valuable insights into a substance's safety, efficacy, and mechanisms of action. These experiments can be categorized into various types based on the experimental setting and
and cells during in-vitro experiments. These solutions mimic the ionic composition of bodily fluids, providing a stable environment for studying physiological processes and drug responses. Objectives 1. Understand the Basics of In-Vitro Pharmacology: Learn about the principles and applications of in-vitro pharmacology. 2.
clinical pharmacology studies, at the 40th Winter Meeting of the Pharmacology and Molecular Mechanisms Group of the European Organization for Research and Treatment of Cancer. Moreover, specific cellular sensitivity tests are described. In addition to monolayer in vitro cell models for the screening of new potential candidate drugs, three ...
Nov 29, 2020 · nerve. In 1878, he published a classic text, Outline of Pharmacology. Schmiedeberg trained most of the men who became professors at other German universities and in several foreign countries.3 Today, there is a pharmacology department in every college of medicine or pharmacy. Experimental pharmacology is done in-vitro and in-vivo. In vivo
Briefly, we summarize the parameters to be taken into account when performing in vitro cell assays, in order to obtain reliable and reproducible results, which was fundamentally discussed by lecturers at the educational course on preclinical and early-phase clinical pharmacology studies, at the 40th Winter Meeting of the Pharmacology and ...