Practical Biology
A collection of experiments that demonstrate biological concepts and processes.


Observing earthworm locomotion

Practical Work for Learning

Published experiments
Investigating an enzyme-controlled reaction: catalase and hydrogen peroxide concentration, class practical or demonstration.
Hydrogen peroxide ( H 2 O 2 ) is a by-product of respiration and is made in all living cells. Hydrogen peroxide is harmful and must be removed as soon as it is produced in the cell. Cells make the enzyme catalase to remove hydrogen peroxide.
This investigation looks at the rate of oxygen production by the catalase in pureed potato as the concentration of hydrogen peroxide varies. The oxygen produced in 30 seconds is collected over water. Then the rate of reaction is calculated.
Lesson organisation
You could run this investigation as a demonstration at two different concentrations, or with groups of students each working with a different concentration of hydrogen peroxide. Individual students may then have time to gather repeat data. Groups of three could work to collect results for 5 different concentrations and rotate the roles of apparatus manipulator, result reader and scribe. Collating and comparing class results allows students to look for anomalous and inconsistent data.
Apparatus and Chemicals
For each group of students:.
Pneumatic trough/ plastic bowl/ access to suitable sink of water
Conical flask, 100 cm 3 , 2
Syringe (2 cm 3 ) to fit the second hole of the rubber bung, 1
Measuring cylinder, 100 cm 3 , 1
Measuring cylinder, 50 cm 3 , 1
Clamp stand, boss and clamp, 2
Stopclock/ stopwatch
For the class – set up by technician/ teacher:
Hydrogen peroxide, range of concentrations, 10 vol, 15 vol, 20 vol, 25 vol, and 30 vol, 2 cm 3 per group of each concentration ( Note 1 )
Pureed potato, fresh, in beaker with syringe to measure at least 20 cm 3 , 20 cm 3 per group per concentration of peroxide investigated ( Note 2 )
Rubber bung, 2-holed, to fit 100 cm 3 conical flasks – delivery tube in one hole (connected to 50 cm rubber tubing)
Health & Safety and Technical notes
Wear eye protection and cover clothing when handling hydrogen peroxide. Wash splashes of pureed potato or peroxide off the skin immediately. Be aware of pressure building up if reaction vessels become blocked. Take care inserting the bung in the conical flask – it needs to be a tight fit, so push and twist the bung in with care.
Read our standard health & safety guidance
1 Hydrogen peroxide: (See CLEAPSS Hazcard) Solutions less than 18 vol are LOW HAZARD. Solutions at concentrations of 18-28 vol are IRRITANT. Take care when removing the cap of the reagent bottle, as gas pressure may have built up inside. Dilute immediately before use and put in a clean brown bottle, because dilution also dilutes the decomposition inhibitor. Keep in brown bottles because hydrogen peroxide degrades faster in the light. Discard all unused solution. Do not return solution to stock bottles, because contaminants may cause decomposition and the stock bottle may explode after a time.
2 Pureed potato may irritate some people’s skin. Make fresh for each lesson, because catalase activity reduces noticeably over 2/3 hours. You might need to add water to make it less viscous and easier to use. Discs of potato react too slowly.
3 If the bubbles from the rubber tubing are too big, insert a glass pipette or glass tubing into the end of the rubber tube.
SAFETY: Wear eye protection and protect clothing from hydrogen peroxide. Rinse splashes of peroxide and pureed potato off the skin as quickly as possible.
Preparation
a Make just enough diluted hydrogen peroxide just before the lesson. Set out in brown bottles ( Note 1 ).
b Make pureed potato fresh for each lesson ( Note 2 ).
c Make up 2-holed bungs as described in apparatus list and in diagram.
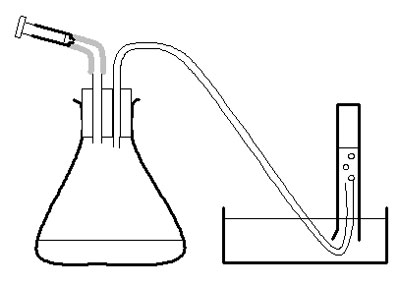
Investigation
d Use the large syringe to measure 20 cm 3 pureed potato into the conical flask.
e Put the bung securely in the flask – twist and push carefully.
f Half-fill the trough, bowl or sink with water.
g Fill the 50 cm 3 measuring cylinder with water. Invert it over the trough of water, with the open end under the surface of the water in the bowl, and with the end of the rubber tubing in the measuring cylinder. Clamp in place.
h Measure 2 cm 3 of hydrogen peroxide into the 2 cm 3 syringe. Put the syringe in place in the bung of the flask, but do not push the plunger straight away.
i Check the rubber tube is safely in the measuring cylinder. Push the plunger on the syringe and immediately start the stopclock.
j After 30 seconds, note the volume of oxygen in the measuring cylinder in a suitable table of results. ( Note 3 .)
k Empty and rinse the conical flask. Measure another 20 cm 3 pureed potato into it. Reassemble the apparatus, refill the measuring cylinder, and repeat from g to j with another concentration of hydrogen peroxide. Use a 100 cm 3 measuring cylinder for concentrations of hydrogen peroxide over 20 vol.
l Calculate the rate of oxygen production in cm 3 /s.
m Plot a graph of rate of oxygen production against concentration of hydrogen peroxide.
Teaching notes
Note the units for measuring the concentration of hydrogen peroxide – these are not SI units. 10 vol hydrogen peroxide will produce 10 cm 3 of oxygen from every cm 3 that decomposes.( Note 1 .)
In this procedure, 2 cm 3 of 10 vol hydrogen peroxide will release 20 cm 3 of oxygen if the reaction goes to completion. 2 cm 3 of liquid are added to the flask each time. So if the apparatus is free of leaks, 22 cm 3 of water should be displaced in the measuring cylinder with 10 vol hydrogen peroxide. Oxygen is soluble in water, but dissolves only slowly in water at normal room temperatures.
Use this information as a check on the practical set-up. Values below 22 cm 3 show that oxygen has escaped, or the hydrogen peroxide has not fully reacted, or the hydrogen peroxide concentration is not as expected. Ask students to explain how values over 22 cm 3 could happen.
An error of ± 0.05 cm 3 in measuring out 30 vol hydrogen peroxide could make an error of ± 1.5 cm 3 in oxygen production.
Liver also contains catalase, but handling offal is more controversial with students and introduces a greater hygiene risk. Also, the reaction is so vigorous that bubbles of mixture can carry pieces of liver into the delivery tube.
If collecting the gas over water is complicated, and you have access to a 100 cm 3 gas syringe, you could collect the gas in that instead. Be sure to clamp the gas syringe securely but carefully.
The reaction is exothermic. Students may notice the heat if they put their hands on the conical flask. How will this affect the results?
Health and safety checked, September 2008
http://www.saps.org.uk/secondary/teaching-resources/293-student-sheet-24-microscale-investigations-with-catalase Microscale investigations with catalase – which has been transcribed onto this site at Investigating catalase activity in different plant tissues.
(Website accessed October 2011)
Practical Biology: science for everyone
Making real science accessible and interesting for all people.
Monday, March 5, 2012
Easy enzyme experiment: potato catalase, 68 comments:.
Thank you so much, helped a lot in Biology

Glad to have helped!

Catalase is an enzyme that catalyzes the transformation of hydrogen peroxide into water and oxygen. This enzyme functions as a natural antioxidant protecting the cell against oxidative damage. This enzyme finds applications in Research and Clinical Chemistry. It also finds diverse industrial applications in textiles, waste treatment, cosmetics and as a disinfectant agent. catalase
How much potato? How much peroxide? Into what size testube? I would like to run this for a class so Im trying to get all materials together. Also, can I simple grate the potatos ahead of time?
Begin with about 40 grams of peeled potato and blend it in about 100ml of water and ice. Mix this well and add 1 ml to a test tube. Test tubes of 10 to 15ml work well. Add several drops of H2O2 to the potato blend in the bottom of the test tube. Exact amounts do not matter, as long as they are relatively close. Yes, you can mash or blend potatoes ahead of time. Blending works best. I really don't know why I used the word "mash" in this post. Hope this helps. -Matt
Oh-ya, be sure to test this ahead of time and adjust volumes as necessary. The experiment works differently with different potatoes. Error on the side of more potatoes. More potato means more foam.
Thanks. I did a test run this morning with straight potato peelings.Slower reaction but good enough. Started with the liquids first and the dropped in the peels, followed by swirling of the tt to get them to the bottom.My peroxide might be a little dated but 3% and ten minures later the tots were being lifted out of the tubes!
Great! Thanks for letting me know. I never have tried it that way but it sounds extremely easy. I'll have to try your method sometime.

Hi, I have recently done an AS level catalase experiment. We cut out thin discs of potato and put them into a 10cm^3 solution of hydrogen peroxide then measured how long it takes for them to rise to the top. Our independent variable was different concentrations of hydrogen peroxide. Our results showed that as the concentration of H2O2 increased, the rate of reaction increased. I was wondering if you could explain the theory of this. Thanks!
The more substrate(H2O2) for the enzyme to react with the faster the reaction will take place. Higher concentration of H2O2 means there is more substrate for catalase to react with.
Hello, what was your research questionf for it if I may know?¿
If you were to plan an experiment to determine the effect of substrate concentration on enzyme activity. What would you write as the method?
Simply vary the concentration of H2O2, which is the substrate. With store bought H2O2, vary the concentration by adding 100% of the H2O2 to potato extract, 75% H2O2, 50%, 25% and 0%, all to different tubes. Create these various H2O2 concentrations by mixing the H2O2 with water.
Which are the dependent, independent and controlled variables?
Dependent is always going to be bubbles produced when H2O2 is added. The independent variable depends on what type of experiment you are running, it could be H202 concentration, amount of potato, temperature, or pH. Control variables also depend on what type of experiment you are running.
what are the conclusion and discussion?
That entirely depends on how you carry out your experiment.
How much time is needed for this process to occur ?
The reaction happens instantly and only takes a few seconds.
LIFE SAVER!!! had to write a stupid report and didnt have any idea where to begin!
Hi Matt I'm just wondering are there any other conditions we can test on catalase. I mean instead of testing the usual temperature, pH and substrate concentration, are there other conditions that can be manipulated to produce an effect result?
Another condition you can modify is buffer concentration. This can be done simply by modifying NaCl concentration the substrate or enzyme is in.
Yo Matt help mout here why does the bubbles from the oxygen produce remain constant and decrease
Not sure what you mean exactly, but bubbles decrease as the substrate of H2O2 decreases.
hey, I am needing the measurements of your foam in this experiment of a class assignment, I need to compare to a similar experiment I would be very grateful if you could give me your results. cheers
What difference would you expect to see in the experiment using varying temperatures ?
This helped me quite a lot for a Biochemistry that I have to do for school. However, the information on altering the PH levels is rather limited. Would it be possible for you to recommend some online sight where I could find more information on this? Please and thank you in advance :)
hi sir/ma'am, do you mind writing down the materials needed for this experiment and how much is needed for the experiment for it to work effectively as i may be using this experiment for year 12 assessment and i want to make sure it works efficiently. thankyou soo much!
Hello, I am wondering if when adding the baking soda I also have to add some type of liquid to get a reaction. I already know that I'm going to add peroxide to one experiment but for the second one I would like to use baking soda. Let me know, thanks :)
Hi, can I ask something.. Is the rate of enzymatic reaction always directly dependent on the enzymatic concentration? Thank you so much :D
Yes it is, although can also be directly dependent on the temperature of the enzyme and the pH level :)
The results are not able to be measured are they? I need results involving numbers but I really liked the experiment. Is there any way I could do it?
Did you have any sources of error?
hey can you tell me some things that i can put in my discussion?
What are the manipulated results?
Hello! I just want to thank you for uploading this, im supposed to do the lab tomorrow however my teacher refused to help me in any sort of way so thank so much! :D
You know PHEOC? What's the 'Problem' for this lab?
please really want to know the enzymes reaction on prepared potato

why blend the potato? is potato not uniform throughout?
What happens if you add Meat or Something else
This was quite informative :-)
Ayanna, Mickeisha thought that this was not as informative
what are the variables in this experiment
ur stupid I hate school
nu u stupyd
What happens if the oxygen is present? Does it effervesce?
Other than a higher temperature what else will increase the reaction rate?
Why do we cut the potatoes
Why must we cut the potato's
The best results come from fresher potatoes! I cut potato in class as students are ready for the potato.
Will putting, for example: an ammonia solution to lower the pH not affect the reaction of hydrogen peroxide and catalase? And does it matter whether the acidic or basic solution is put in before or after adding the hydrogen peroxide?
what are some experimental sources of error which can occur
thank you so much. however i would like to inquire what happens when three test tubes are set up each containing about three slices of a raw potato and in the first test tube distilled water is added, to the second hydrogen peroxide is added and to the third boiled water is added. what is observed in each test tube and explain each observation. thank you.
Catalase, So how many catalase are there in 1 potato?

Hi Dear, i Like Your Blog Very Much..I see Daily Your Blog ,is A Very Useful For me. Other than tuition, science practical SmartLab also provide full scale Science Lab / Practical lessons for students taking their GCE ’O’ / ‘A’ Level exam Visit Now - http://www.smartlab.com.sg/Tuition/
If I process potatoes in a Green Star twin gear juice extractor, low 110 rpm with minuscule heat generation, where is the catalase found, in the potato juice, in the potato juice starch fallout, or in the potato pulp? Which kind potatoes have the greatest amount of catalase?
We just did a lab on enzyme catalase, and found out that the reaction rate for the cold liver /peroxide has faster reaction compared to the warm liver /peroxide,how is that possible?
Hi! If we ran this experiment, how would we be able to measure the amount of oxygen produced by the reaction the combination of potatoes and hydrogen peroxide produce?
Author doing a great huge job, thx very interesting experiments, if somebody what to get more information about enzyme you can find it here
what experiment should I do to test the effect of a change in substrate concentration on the activity of the enzyme, using potatoes?
Found you via Pinterest. This has become a weekly meal in our house. thanks for the recipe.
What evidence do you have that the enzyme is not changed in the reactions and can be used more than once
Gracias 🙏🏿 a student from starehe girls helped
May I ask why the oxidized potatos has weaker ability as a catalyst?
- Cell Biology
A Simple Catalase Experiment Using Potatoes: Some plants such as

Related documents

Add this document to collection(s)
You can add this document to your study collection(s)

Add this document to saved
You can add this document to your saved list
Suggest us how to improve StudyLib
(For complaints, use another form )
Input it if you want to receive answer

Catalase Enzyme Lab

Why enzymes are important
Chances are your biochemistry unit is not a student favorite. I often hear “why are we learning chemistry in biology class?” But to really understand how cells work, students need to understand macromolecules. (If you need some biochemistry lesson ideas, check out this blog post ). Luckily, there are fun labs you can do to help students understand enzymes.
Using catalase to test enzyme efficiency
A common enzyme lab for students to measure the impact of temperature and pH on the efficiency of catalase. Catalase is an enzyme is found in almost all living organisms that breaks down hydrogen peroxide (H2O2) into oxygen and water. Many teachers use raw chicken liver or potato as the source of the catalase. I’ve done both and frankly potato is less stinky and is easier to clean up after. Here is the gist of the lab:
- Students will need: potato puree, tweezers, a beaker full of hydrogen peroxide, and a stopwatch.
- Peel a raw potato and cut it into pieces. Place the potato in the blender and add a small amount of water. Puree until smooth. (One large potato should be enough for 1 class period).
- Note: The potato will turn brown relatively quickly as it comes in contact with the air. Don’t worry! This does not impact the results of the experiment.
- Collect the paper discs out of your hole puncher (or hit up the copy center at your school).
- Using tweezers, have students dip a paper disc in the potato puree. Place the paper in the bottom of the beaker of peroxide and start the stopwatch. As the catalase on the paper disc breaks peroxide into oxygen and water, the disc will float. Have students time how long it takes for the paper to rise.
- Concentration: To show students the impact of concentration on enzyme efficiency, simply water down the potato puree. Do a trial with straight potato, a trial with a 3:1 ratio of potato to water, and a trial with 1:1 ratio of potato to water.
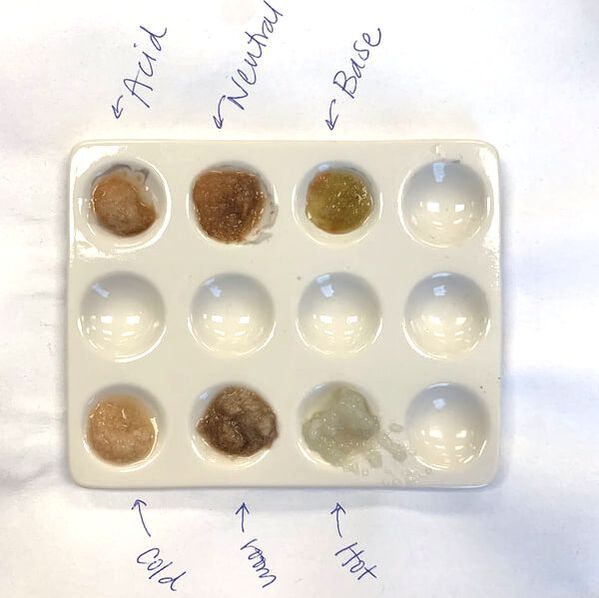
- pH: To show students the impact of pH on enzyme efficiency, have them add a few drops of an acid and a base to the potato purees on a spot plate. Vinegar and bleach are great options. Repeat the experiment and have students determine at which pH catalase works best.
- Option 1: Change the temperature of the peroxide. Place a beaker of peroxide in an ice bath, and another in a warm water bath. This option tends to yield the best results.
- Option 2: Change the temperature of the potato puree. This can be done easily by putting some of the puree in the fridge and some in the microwave (or boil it at home ahead of time). This does not always give the best results because the cold potato can warm up pretty quickly, but still works if you don’t have water baths available.
Troubleshooting Tips
After doing this lab for multiple years, here are some additional tips:
- Have students do multiple trials (at least 3) and take the average. Sometimes they get weird data, so this helps with accuracy.
- If you are testing multiple variables, have students get fresh peroxide before starting the new variable. For example, have students collect all the temperature data, get fresh peroxide, and then collect pH data.
- If the paper disc takes more than 1 minute to rise, tell students that the enzyme is denatured and they can stop timing and move on to the next trial.
- When I first started doing this lab I used petri dishes for all the potato purees and it was a lot of clean up. I recently switched to chemistry spot plates (pictured above) and it made clean up so much easier!
If you would like to purchase a lab write-up, you can find it here:

- Read more about: Biochemistry

Hi, I'm Becca!
Search the site, browse by category.
- Back to School
- Biochemistry
- Body Systems
- Classification
- Classroom Decor
- Classroom Management
- Distance Learning
- End of the School Year
- Experiments
- Field Trips
- For NEW Teachers
- Formative Assessment
- Media in the Classroom
- Microscopes
- Photosynthesis & Respiration
- Plate Tectonics
- Sustainability
- Teacher Tips
- Weather and Climate
Get Freebies!
You might also like....

Teaching DNA Structure and Replication just got easier

10 Atmosphere Lessons for High School

Read Aloud Closure Assignments

Want a fun way to practice science vocabulary? Try out seek and finds!

Privacy Overview

COMMENTS
Cells make the enzyme catalase to remove hydrogen peroxide. This investigation looks at the rate of oxygen production by the catalase in pureed potato as the concentration of hydrogen peroxide varies. The oxygen produced in 30 seconds is collected over water. Then the rate of reaction is calculated.
Using a potato and hydrogen peroxide, we can observe how enzymes like catalase work to perform decomposition, or the breaking down, of other substances. Catalase works to speed up the decomposition of hydrogen peroxide into oxygen and water.
In this practical, students investigate the presence of enzymes in liver, potato and celery by detecting the oxygen gas produced when hydrogen peroxide decomposes. The experiment should take no more than 20–30 minutes.
Potato catalase experiment. The boiled tube (left) produced no bubbles indicating catalase has been degraded by the heat. Room temperature tube (middle) produced the most bubbles indicating catalase is highly functional at this temperature.
Potato catalase experiment. The boiled tube (left) produced no bubbles indicating catalase has been degraded by the heat. Room temperature tube (middle) produced the most bubbles indicating catalase is highly functional at this temperature.
Catalase is an enzyme is found in almost all living organisms that breaks down hydrogen peroxide (H2O2) into oxygen and water. Many teachers use raw chicken liver or potato as the source of the catalase.
In this laboratory exercise, a crude cell extract is prepared from potatoes. Activity of the enzyme, catalase [which catalyzes the reaction 2H 2 O 2 (l) → 2H 2 O (l) + O 2 (g)], is then studied using a simple assay for O 2.
This experiment investigates the effect of pH on catalase activity. and observes the catalytic action of two sources of catalase on hydrogen peroxide. Hypotheses . Hypothesis 1: If the pH is below or above the optimum pH, the rate of the catalytic action of catalase will decrease. Hypothesis 2:Animal cells contain more catalase than plant cells.
The potato puree is filtered thoroughly and quickly by 4-ply cheesecloth which can be obtained from most biological supply houses. • While this experiment will work without buffering the solutions (using water as diluent), the use of a buffered solution will help to maintain catalase activity for a longer period of time before the
The experiment Below is a way to look at how well the enzyme catalase (which is found in high quantities in potatoes) is working. This investigation looks at the rate of oxygen production by the catalase, by collecting the oxygen produced over a 30-second period. Enzymes work quickly –and in fact, catalase might be one of the fastest enzymes